NEW YORK CITY (dpa-AFX) - Arvinas, Inc. (ARVN) and Pfizer Inc. (PFE), Tuesday announced preliminary data from the ongoing Phase 1b portion of the TACTIVE-U sub-study of Vepdegestrant in combination with Abemaciclib in patients with locally advanced or metastatic estrogen receptor positive/human epidermal growth factor receptor 2 negative breast cancer.
The findings revealed that the combination of Aabemaciclib 150 mg twice daily with the recommended Phase 3 monotherapy dose of 200 mg of Vepdegestrant once daily was safe and well-tolerated in the participants. Moreover, it showed no significant drug-drug interaction, and no clinically meaningful effect on Abemaciclib exposure.
The companies added that the combination therapy demonstrated a clinical benefit rate of 62.5 percent, and overall response rate of 26.7 percent in patients previously treated with a CDK4/6 inhibitor.
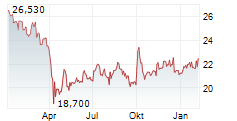
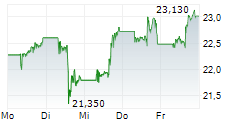
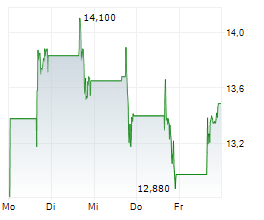
Currently, Arvinas's stock is falling 1.75 percent, to $24.64 on the Nasdaq, while Pfizer's stock is trading at $25.81, down 1.13 percent on the New York Stock Exchange.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News