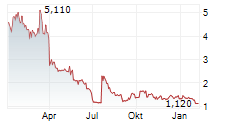
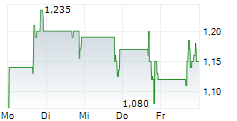
Transaction follows a $5 million non-dilutive credit line from the same trusted partner, further reinforcing Telomir's financial strength and growth potential.
MIAMI, FL / ACCESSWIRE / December 12, 2024 / Telomir Pharmaceuticals, Inc. (Nasdaq:TELO) ("Telomir" or the "Company"), an emerging leader in age-reversal science, today announced it has raised $1 million in equity funding through The Starwood Trust. The investment, structured as a straightforward common stock transaction, was made at $7 per share representing a 20% premium to the closing price on the date of execution and included no warrants. The restricted shares further emphasize The Starwood Trust's confidence in the company's long-term strategy and potential.
This funding builds upon the $5 million non-dilutive line of credit extended by The Starwood Trust earlier this year, which remains undrawn. Together, these financial milestones reflect Telomir's commitment to advancing its innovative pipeline while aiming to maintain a strong financial foundation.
Recent Breakthroughs Highlight Telomir-1's Transformative Potential
Telomir recently announced potentially groundbreaking preclinical results for its lead compound, Telomir-1, confirming age-reversal and longevity benefits, as well as the ability to address Type 2 diabetes at its root cause. Studies showed significant reductions in fasting plasma glucose levels, improved glucose homeostasis, and the reversal of insulin resistance to near pre-diabetic levels.
Unlike current therapies that primarily manage symptoms, Telomir-1 addresses the root causes of aging and disease, including oxidative stress, chronic inflammation, telomere shortening, and regulation of metal overactivity, such as iron and copper dysregulation. This innovative approach positions Telomir-1 as a potential breakthrough therapy across multiple age-related conditions.
Erez Aminov, Chairman and CEO of Telomir, commented, "Securing funding at a premium to the closing price without warrants demonstrates the strength of our financial strategy and our commitment to delivering value to shareholders. These recent preclinical results reaffirm our belief in Telomir-1's ability to redefine how we treat chronic diseases and aging by addressing their root causes."
Expanding Research and Future Directions
Telomir is advancing its exploration in a pipeline of innovative therapies for critical conditions, including:
Progeria Studies: Investigating Telomir-1's effects on telomere stability and accelerated aging in human cell lines and nematode models.
Type 2 Diabetes: Further validating Telomir-1's ability to reverse insulin resistance and improve glucose homeostasis in rodent models.
Alzheimer's Disease: Assessing the molecule's role in mitigating neurodegeneration and cognitive decline.
Cancer Models: Evaluating applications in selective oncology conditions.
Wilson Disease: Targeting copper overload, a hallmark of this rare genetic disorder, through preclinical studies.
Osteoarthritis Models: Exploring Telomir-1's potential to improve joint health and mobility in age-related degenerative and inflammatory conditions.
The global market for age-reversal and age-related therapies offers significant opportunities, with a growing focus on therapies that address the underlying causes of these conditions. Telomir is poised to capitalize on these opportunities as it advances Telomir-1 toward clinical development.
Dr. Itzchak Angel, Chief Scientific Advisor at Telomir, stated, "Telomir-1's potential extends far beyond treating symptoms-it targets the biological mechanisms underlying numerous chronic diseases. By addressing key drivers of cellular aging, inflammation and disease, such as oxidative stress, telomere shortening, and metal overload, Telomir-1 has the potential to fundamentally change the way we approach healthcare."
About Telomir Pharmaceuticals, Inc.
Telomir Pharmaceuticals, Inc. (Nasdaq:TELO) is a pre-clinical stage pharmaceutical company seeking to lead development in age-reversal science. Telomir is focused on the development of Telomir-1, a novel small molecule designed to lengthen the DNA's protective telomere caps, which are crucial in the aging process. Telomir's goal is to explore the potential of Telomir-1 starting with ongoing research in animals and then in humans.
Telomeres are the protective end caps of a chromosome made up of complex between DNA sequences and proteins. As humans age, telomeres shorten, with metal reactivity accelerating the process, which presents humans and pet animals with an increased chance of contracting a number of degenerative and age-related diseases. Telomir's goal is to develop and gain regulatory approval for Telomir-1, proposed to be dosed orally, with the broader aim of promoting longevity and enhancing overall quality of life.
The Nobel Assembly at Karolinska Institute (Sweden) awarded the Nobel Prize in Physiology or Medicine in 2009 for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase.
Telomir-1 is in preclinical development and has not yet been tested in humans. There is no assurance that Telomir-1 will proceed through development or will ultimately receive FDA approval for marketing.
Cautionary Note Regarding Forward-Looking Statements
This press release, statements of Telomir Pharmaceuticals' management or advisors related thereto, and the statements contained in the news story linked in this release contain "forward-looking statements," which are statements other than historical facts made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements may be identified by words such as "aims," "anticipates," "believes," "could," "estimates," "expects," "forecasts," "goal," "intends," "may," "plans," "possible," "potential," "seeks," "will," and variations of these words or similar expressions that are intended to identify forward-looking statements. Any such statements in this press release that are not statements of historical fact may be deemed to be forward-looking statements. These forward-looking statements include, without limitation, statements regarding (i) the anticipated benefits of the preclinical testing results described herein, (ii) anticipated timelines and subject matter for additional preclinical and clinical testing of Telomir-1 and (iii) the potential therapeutic benefits of Telomir-1 generally.
Any forward-looking statements in this press release are based on Telomir's current expectations, estimates and projections only as of the date of this release and are subject to a number of significant risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to, the potential use of the data from our studies, our ability to develop and commercialize Telomir-1 for specific indications and safety of Telomir-1. These and other risks concerning Telomir's programs and operations are described in additional detail in its Annual Report on Form 10-K for the fiscal year ended December 31, 2023, which is on file with the SEC. Telomir explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Contact Information
Helga Moya
info@telomirpharma.com
(786) 396-6723
SOURCE: Telomir Pharmaceuticals, Inc.
View the original press release on accesswire.com