COPENHAGEN (dpa-AFX) - Novo Nordisk A/S (NVO), Thursday announced that the European Medicines Agency's Committee for Medicinal Products for Human Use or CHMP has supported label update for weight-loss drug Ozempic to include kidney disease risk reduction.
The decision was based on findings of the FLOW trial, which assessed the risk reduction from Ozempic therapy, a once-weekly subcutaneous semaglutide, in chronic kidney disease-related events.
The randomized, double-blinded, parallel-grouped, placebo-controlled, superiority trial found that semaglutide 1.0 mg reduced kidney disease risk by 24 percent. Whereas, it cut the risk of major cardiovascular events by 18 percent, and the risk of all-cause mortality by 20 percent.
The Denmark-based company has also filed for a label expansion in the US, and a decision is expected in the first half of 2025.
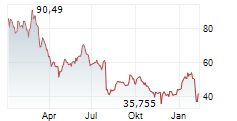
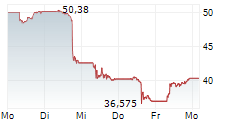
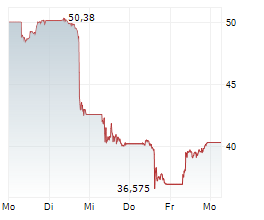
Currently, Novo Nordisk's stock is trading at $110.57, down 1 percent on the New York Stock Exchange.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News