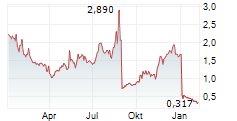
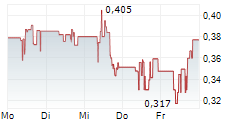
ISELIN, N.J., Dec. 13, 2024 (GLOBE NEWSWIRE) -- Outlook Therapeutics, Inc. (Nasdaq: OTLK), a biopharmaceutical company that achieved regulatory approval in the European Union and the United Kingdom earlier this year for the first authorized use of an ophthalmic formulation of bevacizumab for the treatment of wet age-related macular degeneration (wet AMD), today announced that following an internal strategic review, the management team and Board of Directors have implemented initiatives to streamline the organization, reduce operating expenses and preserve capital, with the goal of maximizing its efforts to commercially launch LYTENAVA (bevacizumab gamma) for the treatment of wet AMD in the European Union (EU) and United Kingdom (UK) and supporting the resubmission of the Biologics License Application (BLA) for ONS-5010/LYTENAVA to the U.S. Food and Drug Administration (FDA).
Lawrence Kenyon, Interim Chief Executive Officer and Chief Financial Officer commented, "In light of the current financial market conditions and the Company's strategic focus on the commercial launch of LYTENAVA (bevacizumab gamma) in Europe, following Marketing Authorization granted by the European Commission in the EU and Marketing Authorization granted by the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, we conducted a strategic review with the goal of preserving capital and extending our cash runway as long as possible. As a result of this process, we identified potential efficiencies and are taking immediate cost-saving measures, including a 23% reduction in our workforce representing $1.4 million in annual savings excluding the costs of the reduction in workforce. As we look ahead, we remain steadfast in our belief in the potential of ONS-5010/LYTENAVA to meet the global needs of retina specialists, patients, and payers and are dedicated to advancing our regulatory and commercial efforts."
The Company recently announced that the National Institute for Health and Care Excellence (NICE) has recommended LYTENAVA (bevacizumab gamma) as an option for the treatment of wet AMD. LYTENAVA (bevacizumab gamma) is the first and only authorized ophthalmic formulation of bevacizumab for use in treating wet AMD in adults in the EU and UK and has an initial 10 years of market exclusivity. Separately, upon receipt of the full efficacy and safety results for the NORSE EIGHT clinical trial in the United States, which are expected in January 2025, Outlook Therapeutics plans to resubmit its BLA application for ONS-5010 in the first quarter of calendar 2025. Previously, the Company announced that ONS-5010 did not meet the pre-specified non-inferiority endpoint at week 8 set forth in the special protocol assessment (SPA) with the FDA in the NORSE EIGHT trial. However, the preliminary data from the trial demonstrated an improvement in vision and the presence of biologic activity, as well as a continued favorable safety profile for ONS-5010.
About ONS-5010 / LYTENAVA (bevacizumab-vikg, bevacizumab gamma)
ONS-5010/LYTENAVA is an ophthalmic formulation of bevacizumab for the treatment of wet AMD. LYTENAVA (bevacizumab gamma) is the subject of a centralized Marketing Authorization granted by the European Commission in the European Union (EU) and Marketing Authorization granted by the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom (UK) for the treatment of wet age-related macular degeneration (wet AMD).
In the United States, ONS-5010/LYTENAVA (bevacizumab-vikg) is investigational and is being evaluated in an ongoing non-inferiority study for the treatment of wet AMD.
Bevacizumab-vikg (bevacizumab gamma in the EU and UK) is a recombinant humanized monoclonal antibody (mAb) that selectively binds with high affinity to all isoforms of human vascular endothelial growth factor (VEGF) and neutralizes VEGF's biologic activity through a steric blocking of the binding of VEGF to its receptors Flt-1 (VEGFR-1) and KDR (VEGFR-2) on the surface of endothelial cells. Following intravitreal injection, the binding of bevacizumab to VEGF prevents the interaction of VEGF with its receptors on the surface of endothelial cells, reducing endothelial cell proliferation, vascular leakage, and new blood vessel formation in the retina.
About Outlook Therapeutics, Inc.
Outlook Therapeutics is a biopharmaceutical company focused on the development and commercialization of ONS-5010/LYTENAVA (bevacizumab-vikg; bevacizumab gamma), for the treatment of retina diseases, including wet AMD. LYTENAVA (bevacizumab gamma) is the first ophthalmic formulation of bevacizumab to receive European Commission and MHRA Marketing Authorization for the treatment of wet AMD. Outlook Therapeutics is working to initiate its commercial launch of LYTENAVA (bevacizumab gamma) in the EU and the UK as a treatment for wet AMD, expected in the first half of calendar 2025. In the United States, ONS-5010/LYTENAVA is investigational, is being evaluated in an ongoing non-inferiority study for the treatment of wet AMD, and if successful, the data may be sufficient for Outlook to resubmit a BLA to the FDA in the United States. If approved in the United States, ONS-5010/LYTENAVA, would be the first approved ophthalmic formulation of bevacizumab for use in retinal indications, including wet AMD.
Forward-Looking Statements
This press release contains forward-looking statements. All statements other than statements of historical facts are "forward-looking statements," including those relating to future events. In some cases, you can identify forward-looking statements by terminology such as "anticipate," "continue," "expect," "may," "potential," "will," or "would" the negative of terms like these or other comparable terminology, and other words or terms of similar meaning. These include, among others, Outlook Therapeutics' ability to achieve projected cost savings in connection with initiatives to streamline the organization, including the reduction in workforce, expectations concerning Outlook Therapeutics' ability to remediate or otherwise resolve deficiencies identified in the CRL issued by the FDA, including plans to resubmit the BLA for ONS-5010, plans for commercial launch of LYTENAVA in the UK and EU and timing thereof, Outlook Therapeutics' commercialization strategy, expectations concerning decisions of regulatory bodies and the timing thereof, the therapeutic potential of LYTENAVA as a treatment of wet AMD, ONS-5010/LYTENAVA's potential as the first FDA-approved ophthalmic formulation of bevacizumab for use in treating retinal indications, including wet AMD, in the United States, and other statements that are not historical fact. Although Outlook Therapeutics believes that it has a reasonable basis for the forward-looking statements contained herein, they are based on current expectations about future events affecting Outlook Therapeutics and are subject to risks, uncertainties and factors relating to its operations and business environment, all of which are difficult to predict and many of which are beyond its control. These risk factors include those risks associated with developing and commercializing pharmaceutical product candidates, risks of conducting clinical trials and risks in obtaining necessary regulatory approvals, the content and timing of decisions by regulatory bodies, the sufficiency of Outlook Therapeutics' resources, as well as those risks detailed in Outlook Therapeutics' filings with the Securities and Exchange Commission (SEC), including the Annual Report on Form 10-K for the fiscal year ended September 30, 2023, filed with the SEC on December 22, 2023, and future quarterly reports Outlook Therapeutics files with the SEC, which include uncertainty of market conditions and future impacts related to macroeconomic factors, including as a result of the ongoing overseas conflicts, high interest rates, inflation and potential future bank failures on the global business environment. These risks may cause actual results to differ materially from those expressed or implied by forward-looking statements in this press release. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Outlook Therapeutics does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise, except as may be required under applicable securities law.
Investor Inquiries:
Jenene Thomas
Chief Executive Officer
JTC Team, LLC
T: 908.824.0775
OTLK@jtcir.com