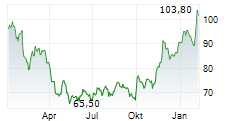
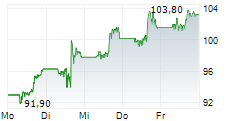
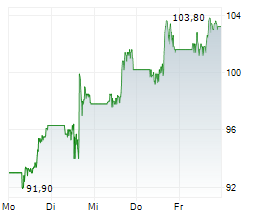
KENILWORTH (NJ) (dpa-AFX) - Merck & Co Inc. (MRK), Thursday reported topline results from two pivotal Phase 3 trials evaluating the company's drug candidate islatravir in combination with its approved HIV drug doravirine for the treatment of adults with virologically suppressed HIV-1 infection.
The trials met primary efficacy criteria for non-inferiority of doravirine/ islatravir (DOR/ISL) combination compared to antiretroviral therapies in adults with virologically suppressed HIV-1. However, the superiority criteria were not met in one of the trials. Safety profiles of DOR/ISL were generally comparable with other therapies in these trials.
The company plans to file these data with regulatory authorities.
A Phase 3 study of DOR/ISL combination in people with HIV who had not previously received treatment is underway.
Islatravir is currently being evaluated in multiple early and late-stage studies in combination with other antiretroviral therapies for the treatment of HIV-1.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News