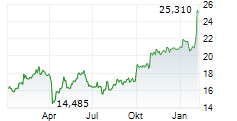
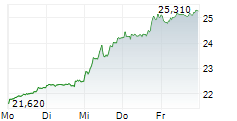
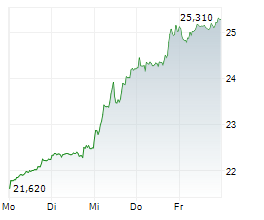
BRUSSELS/FRANKFURT/PARIS (dpa-AFX) - GSK plc (GSK, GSK.L) reported headline results from the FIRST-ENGOT-OV44 phase III trial evaluating Zejula and Jemperli in first line advanced ovarian cancer. The trial met its primary endpoint of PFS demonstrating a statistically significant difference with the addition of dostarlimab to both standard of care carboplatin-paclitaxel chemotherapy and niraparib maintenance, with or without bevacizumab. The company stated that the key secondary endpoint of overall survival did not meet statistical significance.
Hesham Abdullah, Senior Vice President, Global Head Oncology, R&D, GSK, said: 'As part of our focus in gynaecological cancers, we continue to evaluate the potential of this combination and look forward to sharing full results from the trial.'
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News