NEW YORK CITY (dpa-AFX) - Bristol-Myers Squibb Co. (BMY), Monday announced that the European Commission has approved Opdivo plus Yervoy for the first-line treatment of adult patients with microsatellite instability-high or mismatch repair deficient unresectable or metastatic colorectal cancer.
Additionally, Opdivo-based treatment options were also approved for the treatment of multiple tumor types in the European Union.
The approval was based on the findings of the CheckMate -8HW trial, where the combination therapy demonstrated a statistically significant and clinically meaningful improvement in progression-free survival, and cut the risk of disease progression or death by 79 percent.
Upon receiving the approval, the dual immunotherapy combination will be available as a first-line treatment in all 27 member states of the European Union, as well as Iceland, Liechtenstein, and Norway.
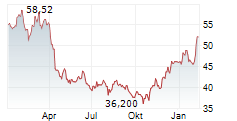
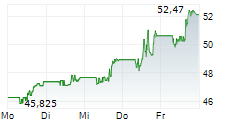
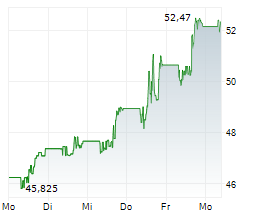
During the pre-market hours, Bristol Myers Squibb's stock is trading at $57.66, up 0.58 percent on the New York Stock Exchange.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News