Bayer AG is making significant strides in the Chinese pharmaceutical market with its prostate cancer medication Nubeqa. The company, in collaboration with Orion Pharma, has submitted an application to Chinese regulatory authorities to expand the drug's approved uses. This development follows compelling Phase III trial results from the Aranote study, which demonstrated that Darolutamid, when combined with hormone therapy, substantially improved radiological progression-free survival in patients with metastatic hormone-sensitive prostate cancer. The study revealed an impressive 46 percent reduction in disease progression or mortality risk compared to placebo treatment.
Market Performance Indicators
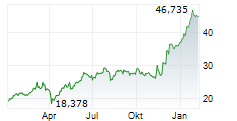
The medication's market presence continues to strengthen, having already secured regulatory approval in more than 80 countries worldwide. Nubeqa achieved a significant milestone by surpassing one billion euros in revenue during the first nine months of the previous year. The potential approval expansion in China would provide healthcare providers with enhanced treatment flexibility, allowing for therapeutic approaches both with and without chemotherapy, potentially further boosting the drug's commercial success in the Asian market.
Ad
Bayer AG Stock: New Analysis - 07 JanuaryFresh Bayer AG information released. What's the impact for investors? Our latest independent report examines recent figures and market trends.
Read our updated Bayer AG analysis...