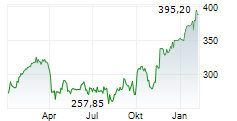
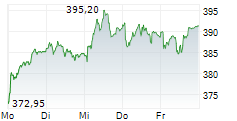
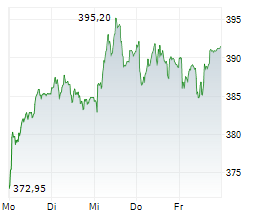
SOUTH SAN FRANCISCO (dpa-AFX) - Roche (RHHBY) Thursday said its whole slide imaging system, Roche Digital Pathology Dx, has received an additional clearance from the Food and Drug Administration (FDA).
The FDA has now given clearance to Roche's VENTANA DP 600 slide scanner, which has 40 times more capacity than VENTANA DP 200 cleared by the regulator in June last year. Roche Digital Pathology Dx now includes the VENTANA DP 200 slide scanner, the VENTANA DP 600 slide scanner, Roche's digital pathology workflow software and a display.
'The VENTANA DP 600 high-capacity slide scanner creates high-resolution, digital images of stained tissue samples that help clinicians diagnose cancer and determine a patient's treatment,' said Jill German, Head of Pathology Lab for Roche Diagnostics.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News