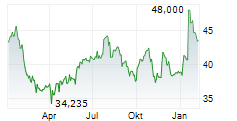
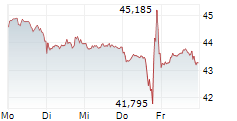
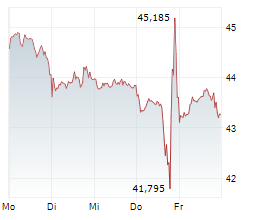
AMSTERDAM (dpa-AFX) - Qiagen (QGEN) announced the U.S. regulatory clearance of the first in a series of QIAstat-Dx Gastrointestinal Panel tests for clinical use.
The clearance by the U.S. Food and Drug Administration marks the second mini syndromic panel in the U.S. and made available for use with QIAstat-Dx systems.
The clearance involves the QIAstat-Dx Gastrointestinal Panel 2 Mini B&V (bacterial and viral) covering five causes of gastrointestinal illness that are recommended by the Infectious Diseases Society of America (IDSA): the bacteria Campylobacter, Salmonella, Shiga-like toxin E.Coli (STEC) and Shigella, along with Norovirus, one of the most common causes of gastrointestinal infection and an important target during the winter season.
A second version of the Gastrointestinal Panel covering five common bacterial pathogens that cause gastrointestinal infections (Campylobacter, Salmonella, STEC, Shigella and Yersinia enterocolitica) is also planned to be submitted to the FDA for clearance in the coming weeks.
QIAGEN also plans to submit QIAstat-Dx Rise, a higher-capacity version of the diagnostic instrument, for FDA clearance in early 2025.
The company noted that the system provides comprehensive testing for up to 160 tests per day using eight Analytical Modules instead of four. QIAstat-Dx syndromic testing with cloud-based connectivity and epidemiological insights is available in more than 100 countries, with more than 4,000 instruments placed worldwide.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News