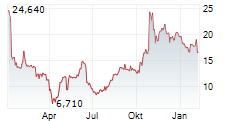
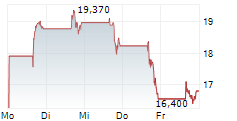
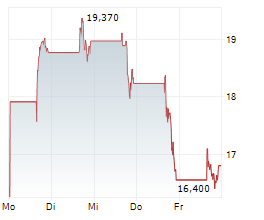
WASHINGTON (dpa-AFX) - Dyne Therapeutics, Inc. (DYN) Friday announced encouraging data from its ongoing Phase 1/2 ACHIEVE trial of DYNE-101 in patients with myotonic dystrophy type 1 (DM1).
Myotonic dystrophy type 1 is a genetic disease that causes progressive muscle weakness and loss.
The company said results from the study showed that DYNE-101 continued to demonstrate a compelling impact on key disease biomarkers.
Dyne plans to initiate a Registrational Expansion Cohort in ACHIEVE, with submission for accelerated approval expected in the first half of 2026.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News