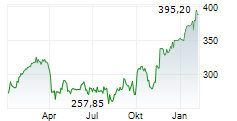
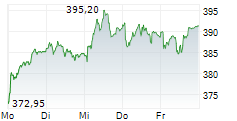
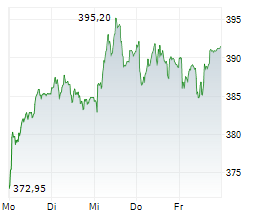
SOUTH SAN FRANCISCO (dpa-AFX) - Roche (RHHBY) said Monday that it has received 510(k) clearance from the U.S. Food and Drug Administration for its highly-sensitive in-situ hybridisation (ISH) test, the VENTANA Kappa and Lambda Dual ISH mRNA Probe Cocktail. The test is designed to help pathologists differentiate a B-cell malignancy from a normal, reactive response to an infection, thus facilitating faster access to treatment. The clearance follows the assay's CE Mark approval in June 2024.
B-cell lymphoma is a type of cancer that typically develops in the lymphatic system. It accounts for approximately 85 percent of non-Hodgkin lymphoma (NHL) cases.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News