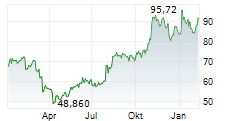
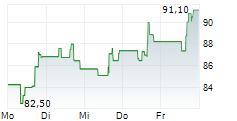
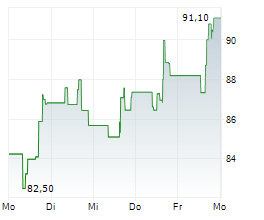
WASHINGTON (dpa-AFX) - Incyte (INCY) and Syndax Pharmaceuticals (SNDX) announced that the U.S. Food and Drug Administration has approved Niktimvo (axatilimab-csfr) in 9 mg and 22 mg vial sizes. The Companies expect product to be available for order in the U.S. in early February.
Niktimvo is approved for the treatment of chronic graft-versus-host disease (GVHD) after failure of at least two prior lines of systemic therapy in adult and pediatric patients weighing at least 40 kg (88.2 lbs).
Niktimvo is the first and only FDA-approved prescription treatment for chronic GVHD that targets CSF-1R to reduce the drivers of inflammation and fibrosis.
The approved dose of Niktimvo for adults and pediatric patients weighing at least 40 kg is 0.3 mg/kg, up to a maximum dose of 35 mg, as an intravenous infusion over 30 minutes every two weeks. Niktimvo will be available for healthcare providers to order through a network of specialty distributors in both 9 mg vial and 22 mg vial sizes to facilitate patient dosing, the companies said.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News