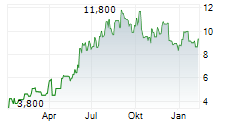
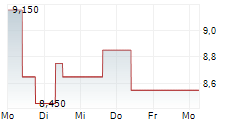
BEIJING (dpa-AFX) - Innovent Biologics Inc. (IVBXF.OB) and Jiangsu Aosaikang Pharmaceutical Co. Ltd. or ASK Pharm announced that China's National Medical Products Administration (NMPA) has approved the New Drug Application of limertinib for the treatment of adult patients with locally advanced or metastatic EGFR T790M-mutated non-small cell lung cancer or NSCLC.
Innovent noted that Limertinib is the 14th product in its commercial portfolio and represents a cutting-edge addition to its strong TKI franchise, offering a precision therapy option to lung cancer patients.
In October 2024, Innovent and ASK Pharm entered into a strategic collaboration and license agreement for limertinib in Mainland China.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News