THOUSAND OAKS (dpa-AFX) - Amgen Inc. (AMGN), Friday announced that the U.S. Food and Drug Administration has approved its Lumakras plus Vectibix combination therapy for the treatment of patients with KRAS G12C-mutated metastatic colorectal cancer.
The approval comes as pivotal Phase 3 CodeBreaK 300 study found that the combination therapy demonstrated superior progression-free survival in the participants compared to the standard treatment.
The study compared Lumakras at doses of 960 mg and 240 mg in combination with Vectibix to investigator's choice of standard of care of trifluridine/tipiracil or regorafenib.
The biotechnology company noted that the combination therapy also met its secondary endpoints by showing favorable overall survival and overall response rate in the participants.
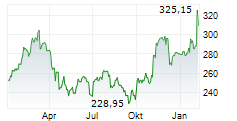
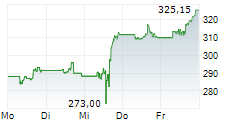
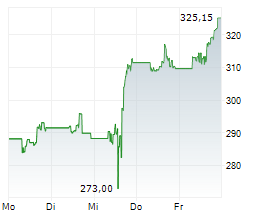
Currently, Amgen's stock is trading at $272.83, up 1.26 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News