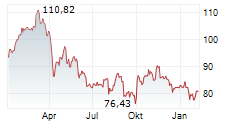
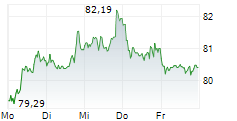
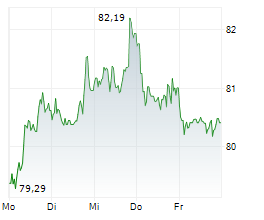
PARIS (dpa-AFX) - Sanofi's Consumer Healthcare division, Opella, announced that the U.S. Food and Drug Administration has lifted a clinical hold on its planned actual use trial (AUT). The trial is aimed at transitioning Cialis (tadalafil) from a prescription-only medication to an over-the-counter option. With this decision, the actual use trial can now proceed, marking Cialis as the first PDE-5 inhibitor to reach the significant milestone.
Cialis (tadalafil) in the US is currently only available with a prescription. Cialis is a tablet taken to treat erectile dysfunction (ED), the signs and symptoms of benign prostatic hyperplasia (BPH), and both ED and the signs and symptoms of BPH.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News