WASHINGTON (dpa-AFX) - Dyne Therapeutics, Inc. (DYN), Tuesday announced that the U.S. Food and Drug Administration has granted Fast Track designation for DYNE-101 for the treatment of myotonic dystrophy type 1, a rare, progressive, genetic disease.
The Fast Track designation is based on clinical data from the company's ongoing Phase 1/2 ACHIEVE trial, demonstrating substantial functional benefits and splicing correction in participants.
The clinical-stage neuromuscular disease company intends to submit for U.S. Accelerated Approval in the first half of 2026.
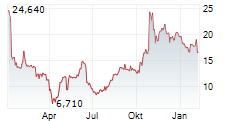
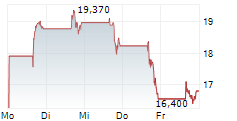
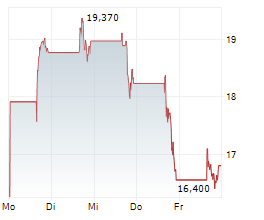
In the pre-market hours, Dyne's stock is trading at $14.01, down 0.82 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News