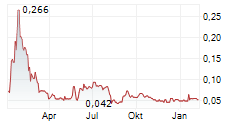
Calgary, Alberta--(Newsfile Corp. - January 23, 2025) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0) ("Hemostemix" or the "Company") is very pleased to announce that its shares will commence trading on the OTCQB under symbol HMTXF today, January 23, 2025, while the Company presents "Welcome to Your Fountain of Youth!" at the Sequire Investor Summit at 11:00 a.m.
"Listing on the OTCQB enables US investors to buy into their 'Fountain of Youth' and longevity, directly. As an investor in HMTXF, my wife and I invested $300,000 in the last round, which closed in November. We're up 600%. I fully expect in the fullness of time that I will be able to sell part of my position to fund my own ACP treatments. As management and directors, we have invested greater than $9.1 Million in Hemostemix's since 2020. We did so because ACP-01 works: it saves limbs from amputation; it regenerates heart function; it regenerates mental acuity. It is clinically relevant; it is statistically significant; it is safe in 498 treatments. That is all detailed in seven studies of 318 subjects, in nine peer review publications. But why wait for a disease process? Why not invest in Your Fountain of Youth today, to obtain longevity and enjoy the quality of life you have today well into your old age? That is why we trademarked Your Fountain of Youth. That is the message I am delivering to investors," Thomas Smeenk, CEO, said.
Trading on the OTCQB generates the following opportunities for Hemostemix:
1. Higher Standards and Transparency
- The OTCQB requires companies to meet specific eligibility criteria, such as maintaining audited financials and being up-to-date with regulatory reporting. This transparency enhances investor confidence in HMTXF.
2. Increased Liquidity
- Companies who are listed on OTCQB often experience higher trading volumes. Increased liquidity makes it easier for investors to buy and sell HMTXF, which can stabilize stock prices.
3. Access to a Broader Investor Base
- Being listed on OTCQB opens doors to a wider pool of investors, including brokers, managed accounts, institutional investors and others who seek more reliable and transparent opportunities like HMTXF.
4. Market Visibility
- OTCQB companies are included in financial data systems like Bloomberg, Reuters, and Yahoo Finance. This increased visibility attracts more investors.
5. Perception of Credibility
- OTCQB is known as the "Venture Market." Listing on the OTCQB signals that the Company is growth-oriented and committed to maintaining the highest standards of public disclosure.
6. Potential for Institutional Interest
- Due to the higher disclosure requirements, OTCQB companies are more likely to attract interest from institutional investors and analysts. This leads to better funding opportunities, liquidity, and broader market support.
7. Enhanced Corporate Governance
- OTCQB listing rules encourage better corporate governance practices, such as, in Hemostemix's case, appointing independent directors and improving shareholder engagement. This governance attracts sophisticated investors.
8. Step Toward Up-Listing
- OTCQB acts as a stepping stone for companies aiming to list on higher exchanges like NASDAQ or the NYSE. By demonstrating compliance with TSXV and OTCQB standards, Hemostemix is staging itself for an up-listing.
Over the next two days, Thomas Smeenk, President & CEO of Hemostemix, will deliver an engaging presentation highlighting the Company's groundbreaking ACP-01 therapy, global scale-up strategy, and collaborations that are creating economic opportunities for clinicians.
About Hemostemix
Founded in 2003, Hemostemix is a clinical-stage biotechnology company recognized for its patented blood-based stem cell therapeutics platform. Hemostemix is focused on scaling angiogenic, neuronal, and cardiomyocyte cell precursors, to treat no-option patients worldwide.
Learn more: www.hemostemix.com.
Contact: Thomas Smeenk, President & CEO - tsmeenk@hemostemix.com | (905) 580-4170
Stock Information: (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0)
Neither the TSX Venture Exchange nor its Regulation Service Provider (as that term is defined under the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Information: This news release contains "forward-looking information" within the meaning of applicable Canadian securities legislation. All statements, other than statements of historical fact, included herein are forward-looking information. In particular, this news release contains forward-looking information in relation to sales of its lead product ACP-01, the commercialization of ACP-01 via the sale of compassionate treatments under Special Access Program. There can be no assurance that such forward-looking information will prove to be accurate. Actual results and future events could differ materially from those anticipated in such forward-looking information. This forward-looking information reflects Hemostemix's current beliefs and is based on information currently available to Hemostemix and on assumptions Hemostemix believes are reasonable. These assumptions include, but are not limited to: the underlying value of Hemostemix and its Common Shares; the successful resolution of the litigation that Hemostemix is pursuing or defending (the "Litigation"); the results of ACP-01 research, trials, studies and analyses, including the analysis being equivalent to or better than previous research, trials or studies; the receipt of all required regulatory approvals for research, trials or studies; the level of activity, market acceptance and market trends in the healthcare sector; the economy generally; consumer interest in Hemostemix's services and products; competition and Hemostemix's competitive advantages; and Hemostemix obtaining satisfactory financing to fund Hemostemix's operations including any research, trials or studies, and any Litigation. Forward-looking information is Subject to known and unknown risks, uncertainties and other factors that may cause the actual results, level of activity, performance or achievements of Hemostemix to be materially different from those expressed or implied by such forward-looking information. Such risks and other factors may include, but are not limited to: the ability of Hemostemix to complete clinical trials, complete a satisfactory analyses and file the results of such analyses to gain regulatory approval of a phase II or phase III clinical trial of ACP-01; potential litigation Hemostemis may face; general business, economic, competitive, political and social uncertainties; general capital market conditions and market prices for securities; delay or failure to receive board or regulatory approvals; the actual results of future operations including the actual results of future research, trials or studies; competition; changes in legislation affecting Hemostemix; the timing and availability of external financing on acceptable terms; long-term capital requirements and future developments in Hemostemix's markets and the markets in which it expects to compete; lack of qualified, skilled labour or loss of key individuals; and risks related to the COVID-19 pandemic including various recommendations, orders and measures of governmental authorities to try to limit the pandemic, including travel restrictions, border closures, non-essential business closures service disruptions, quarantines, self-isolations, shelters-in-place and social distancing, disruptions to markets, disruptions to economic activity and financings, disruptions to supply chains and sales channels, and a deterioration of general economic conditions including a possible national or global recession or depression; the potential impact that the COVID-19 pandemic may have on Hemostemix which may include a decreased demand for the services that Hemostemix offers; and a deterioration of financial markets that could limit Hemostemix's ability to obtain external financing. A description of additional risk factors that may cause actual results to differ materially from forward-looking information can be found in Hemostemix's disclosure documents on the SEDAR website at www.sedarplus.ca. Although Hemostemix has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking information, there may be other factors that cause results not to be as anticipated, estimated or intended. Readers are cautioned that the foregoing list of factors is not exhaustive. Readers are further cautioned not to place undue reliance on forward-looking information as there can be no assurance that the plans, intentions or expectations upon which they are placed will occur. Forward-looking information contained in this news release is expressly qualified by this cautionary statement. The forward-looking information contained in this news release represents the expectations of Hemostemix as of the date of this news release and, accordingly, it is Subject to change after such date. However, Hemostemix expressly disclaims any intention or obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as expressly required by applicable securities law.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/238189
SOURCE: Hemostemix Inc.