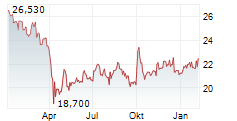
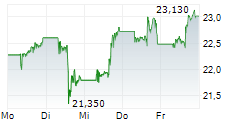
NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) announced positive results from the Phase 3 BREAKWATER trial evaluating BRAFTOVI (encorafenib) in combination with cetuximab (marketed as ERBITUX) and mFOLFOX6 (fluorouracil, leucovorin, and oxaliplatin) in patients with metastatic colorectal cancer (mCRC) with a BRAF V600E mutation.
The results from the Phase 3 trial showed objective response rate of 61% with Pfizer's BRAFTOVI combination regimen compared to 40% with investigator's choice of chemotherapy, representing a doubling of the odds of achieving an objective response.
The estimated median duration of response as assessed by BICR was 13.9 months with BRAFTOVI plus cetuximab and mFOLFOX6 and 11.1 months with chemotherapy with or without bevacizumab. Of patients on BRAFTOVI plus cetuximab and mFOLFOX6, 22.4% (n=15) had a response lasting 12 months or longer, compared to 11.4% with chemotherapy with or without bevacizumab. The median time to response as assessed by BICR was 7.1 weeks with BRAFTOVI plus cetuximab and mFOLFOX6 and 7.3 weeks with chemotherapy with or without bevacizumab.
The company noted that overall survival data were immature at the time of this analysis but demonstrated a promising trend in favor of BRAFTOVI plus cetuximab and mFOLFOX6 compared to patients receiving chemotherapy with or without bevacizumab. Median OS with BRAFTOVI plus cetuximab with chemotherapy was not estimable and 14.6 months with chemotherapy with or without bevacizumab. The BREAKWATER trial is ongoing for OS and progression-free survival (PFS), with PFS results expected in 2025.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News