SOUTH SAN FRANCISCO (dpa-AFX) - Genentech, a member of the Roche Group (RHHBY), announced that the U.S. Food and Drug Administration has approved Susvimo (ranibizumab injection) 100 mg/mL for the treatment of diabetic macular edema (DME), a leading cause of vision loss in adults with diabetes, affecting more than 29 million adults worldwide. Susvimo is now available to U.S. retina specialists and their patients with DME.
Susvimo was first approved by the FDA for the treatment of wet, or neovascular age-related macular degeneration (AMD) in 2021.
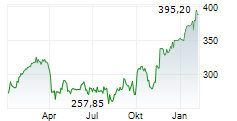
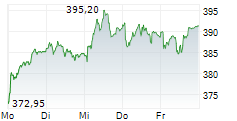
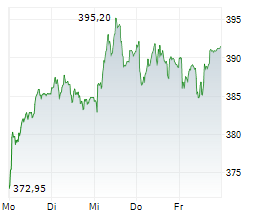
RHHBY closed Tuesday's regular trading at $39.62 up $0.30 or 0.76%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News