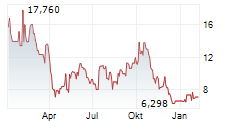
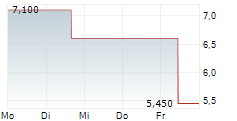
WASHINGTON (dpa-AFX) - Adicet Bio, Inc. (ACET), a clinical stage biotechnology company, Wednesday said it received Fast Track Designation from the U.S. Food and Drug Administration (FDA) for ADI-001 for the treatment of refractory systemic lupus erythematosus (SLE) with extrarenal involvement.
The Company is evaluating ADI-001 across six autoimmune indications. Patient enrollment is ongoing in the Phase 1 study of ADI-001 for the treatment of lupus nephritis (LN). Patient enrollment in SLE, systemic sclerosis (SSc), idiopathic inflammatory myopathy, and stiff person syndrome (SPS) is expected to be initiated in the first quarter of 2025.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News