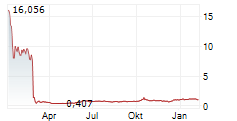
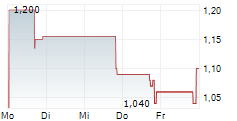
IRVINE, Calif., Feb. 07, 2025 (GLOBE NEWSWIRE) -- AEON Biopharma, Inc. (NYSE: AEON) (the "Company"), a clinical-stage biopharmaceutical company focused on developing a botulinum toxin complex under a 351(k) biosimilar pathway, today announced that on February 3, 2025, AEON received a notice (the "Notice") from the NYSE American LLC (the "NYSE American") stating that the Company is not in compliance with Section 1003(a)(i) of the NYSE American Company Guide (the "Company Guide") requiring a stockholders' equity of $2.0 million or more if it has reported losses from continued operations and/or net losses in two of its three most recent fiscal years, as a result of the Company's reported stockholders' deficit of $32.1 million at September 30, 2024, and losses from continued operations and/or net losses in two of its most recent fiscal years ended December 31, 2023, as reflected in the Company's Annual Report on Form 10-K/A filed May 14, 2024. The Notice also indicates that the Company is also not currently eligible for any exemption in Section 1003(a) of the Company Guide.
In connection with its non-compliance with Section 1003(a)(i), the Company must submit a plan (the "Plan") to the NYSE Regulation by March 5, 2025, advising of actions it has taken or will take to regain compliance with the continued listing standards by August 3, 2026. If NYSE Regulation determines to accept the Plan, the Company will be notified in writing and will be subject to periodic reviews, including quarterly monitoring for compliance with the Plan. If the Company does not submit a plan or if the Plan is not accepted, NYSE American will commence delisting proceedings. Furthermore, if the Plan is accepted but the Company is not in compliance with the continued listing standards by August 3, 2026, or if the Company does not make progress consistent with the Plan, NYSE American will initiate delisting proceedings as appropriate. The Company may appeal a staff delisting determination in accordance with Section 1010 and Part 12 of the Company Guide.
The Notice has no immediate impact on the listing of the Company's shares of common stock, par value $0.0001 per share (the "Common Stock"), which will continue to be listed and traded on the NYSE American during this period, subject to the Company's compliance with the other listing requirements of the NYSE American. The Common Stock will continue to trade under the symbol "AEON", but will have an added designation of ".BC" to indicate the status of the Common Stock are "below compliance". The Notice does not affect the Company's ongoing business operations or its reporting requirements with the Securities and Exchange Commission.
The Company intends to fully comply with NYSE American's requests and will submit its Plan accordingly.
About AEON Biopharma
AEON is a clinical stage biopharmaceutical company focused on developing its proprietary botulinum toxin complex, ABP-450 (prabotulinumtoxinA) injection, or ABP-450, for debilitating medical conditions, with an initial focus on the neurosciences market. ABP-450 is the same botulinum toxin complex that is currently approved and marketed for cosmetic indications by Evolus under the name Jeuveau. ABP-450 is manufactured by Daewoong in compliance with current Good Manufacturing Practice, or cGMP, in a facility that has been approved by the U.S. Food and Drug Administration, Health Canada and European Medicines Agency. The product is approved as a biosimilar in Mexico and India. AEON has exclusive development and distribution rights for therapeutic indications of ABP-450 in the United States, Canada, the European Union, the United Kingdom, and certain other international territories. The Company has built a highly experienced management team with specific experience in biopharmaceutical and botulinum toxin development and commercialization. To learn more about AEON, visit www.aeonbiopharma.com.
Forward-Looking Statements
The foregoing material may contain "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. Forward-looking statements include all statements that do not relate solely to historical or current facts, including without limitation statements regarding the Company's product development and business prospects, and can be identified by the use of words such as "may," "will," "expect," "project," "estimate," "anticipate," "plan," "believe," "potential," "should," "continue" or the negative versions of those words or other comparable words. Forward-looking statements are not guarantees of future actions or performance. These forward-looking statements are based on information currently available to the Company and its current plans or expectations and are subject to a number of risks and uncertainties that could significantly affect current plans. Should one or more of these risks or uncertainties materialize, or the underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended, or planned. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee future results, performance, or achievements. Except as required by applicable law, including the security laws of the United States, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results.
Factors that may cause actual results to differ materially from current expectations include, but are not limited to: (i) the outcome of any legal proceedings that may be instituted against AEON or others; (ii) AEON's future capital requirements; (iii) AEON's ability to raise financing in the future; (iv) AEON's ability to continue to meet continued stock exchange listing standards; (v) the possibility that AEON may be adversely affected by other economic, business, regulatory, and/or competitive factors; (vi) the Company's ability to present a Plan that will be accepted by NYSE Regulation; and (vii) other risks and uncertainties set forth in the section entitled "Risk Factors" and "Cautionary Note Regarding Forward-Looking Statements" in the Company's filings with the SEC, which are available on the SEC's website at www.sec.gov.
Contacts
Investor Contact:
Corey Davis, Ph.D.
LifeSci Advisors
+1 212 915 2577
cdavis@lifesciadvisors.com
Source: AEON Biopharma