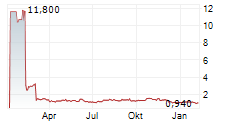
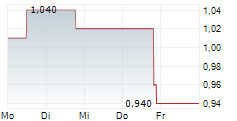
Following DSMB recommendation, the Company has voluntarily paused enrollment and dosing in the BEACON-IPF Phase 2b trial and will monitor current patients while data is reviewed
SOUTH SAN FRANCISCO, Calif., Feb. 07, 2025 (GLOBE NEWSWIRE) -- Pliant Therapeutics, Inc. (Nasdaq: PLRX) today announced that following a prespecified data review and recommendations by the trial's independent Data Safety Monitoring Board (DSMB), the Company has voluntarily paused enrollment and dosing in the ongoing BEACON-IPF Phase 2b trial of bexotegrast in patients with idiopathic pulmonary fibrosis (IPF). Patients currently enrolled in BEACON-IPF will remain in the trial.
Enrollment and dosing have been paused while data is reviewed to understand the DSMB's rationale for their recommendation. Blinding of the study will be maintained to preserve trial integrity. The Company has informed BEACON-IPF clinical trial investigators and is in the process of informing global regulatory authorities.
About Pliant Therapeutics, Inc.
Pliant Therapeutics is a late-stage biopharmaceutical company and leader in the discovery and development of novel therapeutics for the treatment of fibrotic diseases. Pliant's lead product candidate, bexotegrast (PLN-74809), is an oral, small molecule, dual selective inhibitor of avß6 and avß1 integrins that is in development in the lead indication for the treatment of idiopathic pulmonary fibrosis, or IPF. Bexotegrast has received Fast Track Designation and Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) and Orphan Drug Designation from the European Medicines Agency in IPF. Pliant has initiated BEACON-IPF, an adaptive Phase 2b/3 trial of bexotegrast in IPF. Pliant is conducting a Phase 1 study for its third clinical program, PLN-101095, a small molecule, dual-selective inhibitor of avß8 and avß1 integrins, that is being developed for the treatment of solid tumors. In addition, Pliant has received regulatory clearance for the conduct of a Phase 1 study of PLN-101325, a monoclonal antibody agonist of integrin a7ß1 targeting muscular dystrophies.
For additional information, please visit: www.PliantRx.com. Follow us on social media X, LinkedIn, and Facebook.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "anticipate," "estimate," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. These statements include those regarding the Company's and the DSMB's further review and analysis of data, interactions with the DSMB and global regulatory authorities, and the Company's current and future plans for bexotegrast and the BEACON-IPF clinical trial. Because such statements deal with future events and are based on our current expectations, they are subject to various risks and uncertainties and actual results, performance or achievements of Pliant Therapeutics could differ materially from those described in or implied by the statements in this press release. These forward-looking statements are subject to risks and uncertainties, including those related to the development and commercialization of our product candidates, including any delays in our ongoing or planned preclinical or clinical trials, the risks inherent in the drug development process, the risks regarding the accuracy of our estimates of expenses and timing of development, and our capital requirements and the need for additional financing, including the availability of additional term loans under our loan facility. These and additional risks are discussed in the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our Quarterly Report on Form 10-Q for the period ended September 30, 2024, which is available on the SEC's website at www.sec.gov. Unless otherwise noted, Pliant is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
Investor and Media Contact:
Christopher Keenan
Vice President, Investor Relations and Corporate Communications
Pliant Therapeutics, Inc.
ir@pliantrx.com