University of Manitoba independent research verifies that RuvidarTM is more effective than acyclovir in the inactivation of Herpes Simplex Viruses post infection.
TORONTO, ON / ACCESS Newswire / February 10, 2025 / Theralase® Technologies Inc. ("Theralase®" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that independent research conducted at the University of Manitoba has demonstrated that non-light activated RuvidarTM is much more effective in the inactivation of Herpes Simplex Viruses ("HSV") post infection than the gold standard treatment acyclovir.
Infectious agents account for millions of deaths every year1. Currently, the most effective way to protect against infection involve the use of vaccines and anti-microbials. Vaccines are known to be useful, when administered prior to infection; whereas, antibiotics and anti-virals are most useful after infection or before immunity to a vaccine has had time to develop.
The primary disadvantages of vaccines are that knowledge of the agent is required in advance to manufacture an effective vaccine and substantial time is needed to produce relevant vaccines. Furthermore, the developed vaccine may not match the eventual strain that circulates2.
A growing number of anti-viral agents have been developed and some are effective against numerous viruses; however, because viruses replicate and many lack genome proof-reading capabilities, resistance to the anti-viral agent may develop rapidly3,4,5.
HSV are large double-stranded DNA viruses that infect more than 90% of the human population and can establish life-long latency in human hosts.6 Currently, effective FDA approved anti-herpetic drugs include acyclovir and later-generation derivatives (penciclovir, valacyclovir, famciclovir and ganciclovir), which inhibit viral DNA synthesis.
In previous work, Dr. Kevin Coombs, a professor of virology at the University of Manitoba demonstrated that the small molecule, RuvidarTM could inhibit numerous pathogenic human viruses, when added to solutions of viruses, both with and without light-activation. In these latest experiments, Dr. Coombs evaluated the ability of RuvidarTM to restrict HSV-1 replication in Vero cells, both by itself and in combination with acyclovir in the absence of light-activation to mimic deep tissue application.
Light-activated RuvidarTM has been previously demonstrated to be even more effective in the inactivation of HSV versus non-light-activated RuvidarTM.
RuvidarTM successfully inhibited HSV-1 replication at significantly lower concentrations and more effectively than did the gold standard acyclovir alone. Dr. Coombs also discovered additive and synergistic, anti-HSV-1 effects, when combinational therapy was tested.
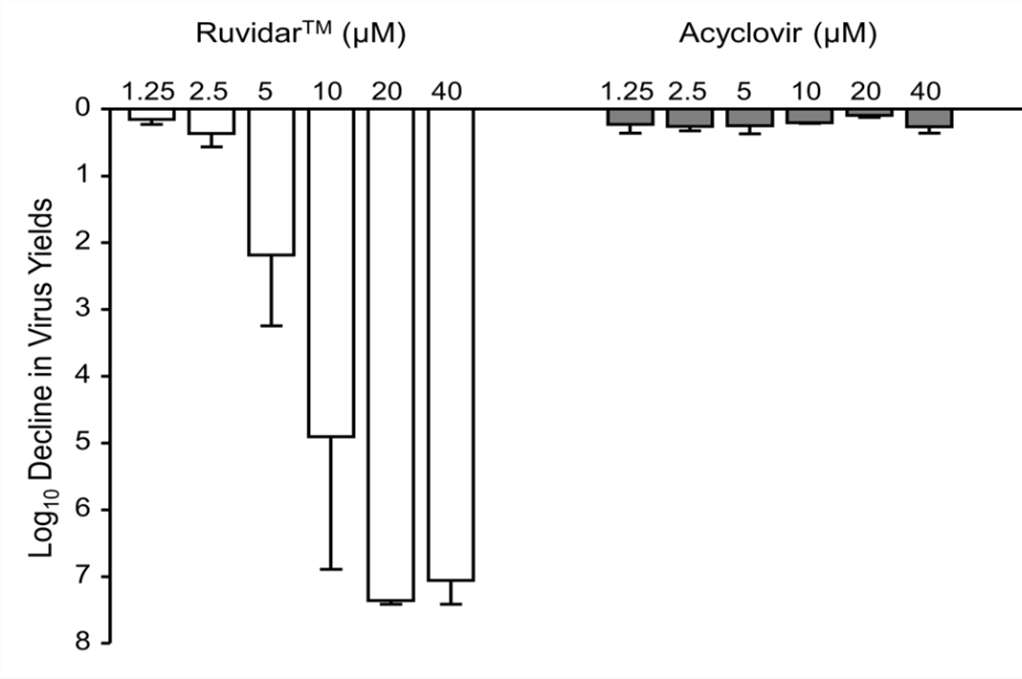
Figure 1. Effects of RuvidarTM versus acyclovir on HSV-1 yields when added 24 hours post infection ("hpi"). Vero cells were infected with HSV-1 at Multiplicity of infection ("MOI") (the number of virions that are added per cell during infection) ~ 1.5, incubated for 24 hours, then treated at 24 hpi with indicated concentrations of drugs for an additional 44 hours. Virus yields were then determined and reductions in virus yields compared to non-treated controls. Error bars represent the Standard Error of Mean from at least three replicates.
Kevin Coombs, B.A., M.A., Ph.D., professor of medical microbiology and infectious diseases at the Max Rady College of Medicine, University of Manitoba (retired) stated, "I have been very impressed in the numerous experiments I have conducted with the ability of both light-activated and non-light-activated RuvidarTM to inactivate numerous viruses. In my latest research, RuvidarTM has been more effective than the gold standard acyclovir in the inactivation of HSV, post infection. Since the majority of the world population is currently infected with one form or another of HSV, RuvidarTM could be game changing as a therapeutic in the treatment of HSV lesions."
Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer, Theralase® stated, "Kevin's work has been instrumental in helping to uncover the efficacy of RuvidarTM in the inactivation of numerous enveloped and non-enveloped viruses. This work will lay the groundwork for both vaccines and therapeutics in the inactivation of viruses that could be used as a platform to prevent and treat the next global pandemic."
Roger DuMoulin-White, B.Sc., P.Eng, Pro.Dir., President and Chief Executive Officer, Theralase® stated, "Based on Kevin's ground-breaking work, Theralase® plans to develop a vaccine and therapeutic for the prevention and treatment of HSV. Preclinical development is currently underway, with clinical development to commence thereafter."
1 www.who.int/data/gho/data/ themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death
2 Chan, M.C.W., Wang, M.H., Chen, Z.G., Hui, D.S.C., Kwok, A.K., Yeung, A.C.M., Liu, K.M., Yeoh, Y.K., Lee, N., Chan, P.K.S., 2018. Frequent genetic mismatch between vaccine strains and circulating seasonal Influenza viruses, Hong Kong, China, 1996-2012. Emerging Infect. Dis. 24, 1825-1834.
3 Colman, P.M., 2009. New antivirals and drug resistance. Annu. Rev. Biochem. 78, 95-118.
4 Krol, E., Rychowska, M., Szewczyk, B., 2014. Antivirals - current trends in fighting influenza. Acta Biochim. Pol. 61, 495-504.
5 Monto, A.S., McKimm-Breschkin, J.L., Macken, C., Hampson, A.W., Hay, A., Klimov, A., Tashiro, M., Webster, R.G., Aymard, M., Hayden, F.G., Zambon, M., 2006. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Ch 50, 2395-2402.
6 Herpesviridae - Wikipedia
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedarplus.ca
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements:
This news release contains Forward-Looking Statements ("FLS") within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. FLS may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of the Company's management regarding future research, development and commercialization of the Company's small molecules; their drug formulations; preclinical research; clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations; the risk that access to sufficient capital to fund the Company's operations may not be available on terms that are commercially favorable to the Company or at all; the risk that the Company's small molecule and drug formulations may not be effective against the diseases tested in its clinical studies; the risk that the Company fails to comply with the terms of license agreements with third parties and as a result loses the right to use key intellectual property in its business; the Company's ability to protect its intellectual property; the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these FLS, which are not a guarantee of future performance. There can be no assurance that FLS will prove to be accurate as such FLS involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the FLS.
Although the FLS contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these FLS.
All FLS are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such FLS.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer X 224
khachey@theralase.com
SOURCE: Theralase Technologies, Inc.
View the original press release on ACCESS Newswire