NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) Wednesday announced the FDA approval of its supplemental Biologics License Application or sBLA for ADCETRIS otherwise known as brentuximab vedotin combined with lenalidomide and a rituximab product.
This treatment is for adults with relapsed or refractory large B-cell lymphoma or LBCL, including diffuse large B-cell lymphoma or DLBCL and high-grade B-cell lymphoma or HGBL, after at least two prior systemic therapies. It is specifically for patients ineligible for autologous stem cell transplantation or auto-HSCT or CAR T-cell therapy.
The FDA approval is supported by Phase 3 ECHELON-3 study data, which showed a significant improvement in overall survival or OS for relapsed/refractory DLBCL patients treated with ADCETRIS plus lenalidomide and rituximab. The study included heavily pre-treated patients, including those previously treated with CAR-T therapy, and demonstrated a survival benefit regardless of CD30 expression.
LBCL, a type of NHL, affects B lymphocytes, key immune cells. DLBCL is its most common and aggressive form, with over 25,000 U.S. cases yearly. Up to 40% relapse or become refractory, and 3,500+ need third-line therapy. Despite advances like bispecifics or CAR-T, a high unmet need remains.
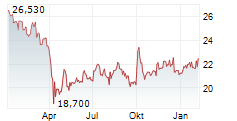
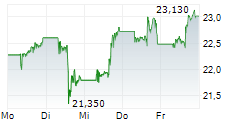
PFE is currently trading at $25.58 or 0.18% higher on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News