NEW BRUNSWICK (dpa-AFX) - The vaccine candidate of Sanofi (SNYNF) and Johnson & Johnson (JNJ) for extraintestinal pathogenic E. coli was not found to be effective at preventing invasive E. coli disease (IED), an interim analysis of the E.mbrace phase 3 study showed, Sanofi said on Thursday.
Following the findings from review conducted by an independent data monitoring committee, the companies are discontinuing the E.mbrace study.
As per the agreement between Sanofi and Janssen Pharmaceuticals, Inc., a Johnson & Johnson company, signed in October 2023, the companies had agreed to share research and development costs of the vaccine candidate.
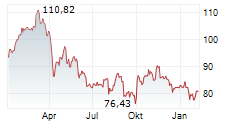
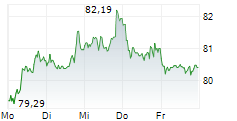
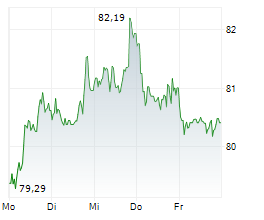
As a result of ending the study, Sanofi has recorded an impairment charge before tax of $250 million in the fourth quarter of fiscal 2024. This reduces the company's full-year EPS reported last month to 4.44 euros from the previous 4.59 euros. However, the company noted that adjusted EPS and fiscal 2025 outlook do not impact from this.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News