DAS/ DIE FOLGENDE(N) INSTRUMENT(E) IST/ SIND AB SOFORT AUSGESETZT:
THE FOLLOWING INSTRUMENT(S) IS/ ARE SUSPENDED WITH IMMEDIATE EFFECT:
INSTRUMENT NAME KUERZEL/SHORTCODE ISIN BIS/UNTIL
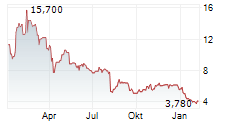
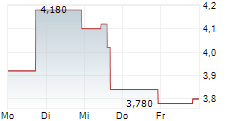
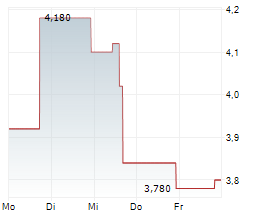
EVOLUS INC. DL-,00001 EVL US30052C1071 BAW/UFN
THE FOLLOWING INSTRUMENT(S) IS/ ARE SUSPENDED WITH IMMEDIATE EFFECT:
INSTRUMENT NAME KUERZEL/SHORTCODE ISIN BIS/UNTIL
EVOLUS INC. DL-,00001 EVL US30052C1071 BAW/UFN
© 2025 Xetra Newsboard