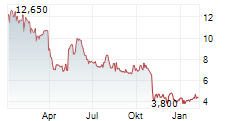
PETAH TIKVA (dpa-AFX) - Alvotech (ALVO) and Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (TEVA), announced that the FDA has accepted for review a Biologics License Application for AVT06, Alvotech's proposed biosimilar to Eylea, a biologic used to treat eye disorders. The process to obtain regulatory approval is anticipated to be completed in the fourth quarter of 2025.
In January 2024, Alvotech announced positive top-line results from a confirmatory clinical study comparing the efficacy, safety, and immunogenicity of AVT06 with Eylea in patients with neovascular AMD.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News