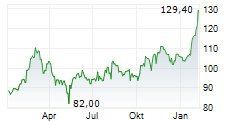
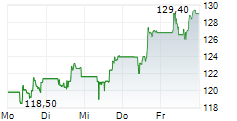
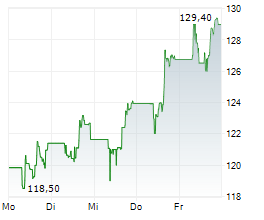
FOSTER CITY (dpa-AFX) - Gilead Sciences (GILD) announced that the FDA has accepted its New Drug Application submissions for lenacapavir-the twice-yearly injectable HIV-1 capsid inhibitor-for the prevention of HIV as pre-exposure prophylaxis. The FDA will review the applications under priority review and has assigned a PDUFA target action date of June 19, 2025. The NDAs are based on data from the Phase 3 PURPOSE 1 and PURPOSE 2 trials conducted by Gilead.
The company noted that the use of lenacapavir for the prevention of HIV is investigational and is not approved anywhere globally.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News