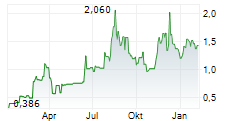
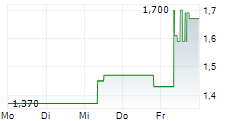
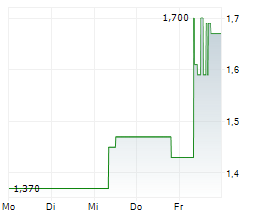
BEIJING (dpa-AFX) - Ascletis Pharma Inc. (ASCLF.PK) Wednesday reported positive interim results from the Phase 1b study of ASC30 for the treatment of obesity.
The Phase Ib multiple ascending dose (MAD) study consists of 3 cohorts. In each cohort, eight patients were treated with ASC30 and 2 were on placebo. The average daily dose over the 28-day treatment period was 9.25 mg ASC30 for cohort 1, and 18 mg for cohort 2.
Interim results from the study showed mean body weight reductions from baselines of 4.3% and 6.3% for cohorts 1 and 2, respectively, after the 28-day treatment period. Further, the drug candidate was well tolerated with no reports of serious adverse events.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News