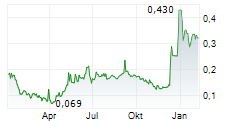
The clinical Phase I study conducted in the NEX-22 project with good results and focus on the evaluation with Novo Nordisk has provided a good starting point for 2025.
Significant events during the fourth quarter 2024
- In October, Nanexa announced that the dosing of the last patient was completed in the Phase I study with long-acting depot formulation of the GLP-1-analogue liraglutide with PharmaShell (NEX-22).
- Nanexa announced in early November that the company's Phase I study for NEX-22 in type 2-diabetes was completed for all patients.
- In late November, Nanexa announced positive results in the company's phase I study for NEX-22, long-acting GLP1, in type 2-diabetes. The study evaluates a depot formulation of the GLP-1 analogue liraglutide for once-monthly dosing.
Significant events after the end of the period
- In January, Nanexa announced that the company has decided to carry out a directed share issue, deviating from existing shareholders' preferential rights, of units amounting to 35 MSEK in two steps. Furthermore, it was announced that the company has taken loans totaling 20 MSEK.
- Nanexa AB announced in January that the company is calling shareholders to an Extraordinary General Meeting on February 13, 2025, in connection with the above-mentioned issue.
- In January Nanexa announced that the Phase I-study with NEX-22, the company's one-month formulation of liraglutide, will resume with further dose escalation with an estimated start in the first quarter of 2025. The study has now received regulatory approval for the administration of 30 mg liraglutide in an additional dose group.
- At the Extraordinary General Meeting on February 13, it was decided that the directed share issue would be completed.
Summary of the reporting period 1 October - 31 December 2024
- Turnover amounted to: TSEK 4,517 (6,816)
- Operating profit (EBIT) amounted to: TSEK -12,025 (-51,367)
- Profit after tax amounted to: TSEK -11,631 (-51,150)
- Earnings per share amounted to: SEK -0.09 (-0.52)
- Cash flow for the period amounted to: TSEK -18,718 (44,599)
- Cash and cash equivalents at end of period: TSEK 10,292 (65,168)
Summary of the reporting period 1 January - 31 December 2024
- Turnover amounted to: TSEK 24,361 (29,327)
- Operating profit (EBIT) amounted to: TSEK -26,062 (-76,625)
- Profit after tax amounted to: TSEK -24,905 (-76,398)
- Earnings per share amounted to: SEK -0.18 (-1.09)
- Cash flow for the period amounted to: TSEK -54,877 (-16,014)
- Cash and cash equivalents at end of period: TSEK 10,292 (65,168)
- The Board of Directors proposes that no dividend will be paid for the financial year 2024
Figures in brackets refer to the corresponding period in the previous year.
The entire report is available on the company's website: https://nanexa.com/en/financial-reports/.
Report commentary, February 19 at 11:00am CET
A live commentary with CEO David Westberg and chairman Göran Ando will take place on February 19 at 11:00am via Infront Direkt Studios and viewers will have the opportunity to ask questions via chat. The presentation will be held in Swedish.
The report commentary will be available via this link.
The report comment will also be published on Nanexa's website afterwards.
For additional information, please contact:
David Westberg - CEO, Nanexa AB (publ)
Phone: +46 70 942 83 03
Email: david.westberg@nanexa.se
www.nanexa.com
The company's Certified Adviser is Carnegie Investment Bank AB (publ).
About Nanexa AB (publ)
Nanexa is a pharmaceutical company developing injectable drug products based on the proprietary and innovative drug delivery system PharmaShell® - the high drug load delivery system enabling the next generation long-acting injectables through atomic layer precision. Nanexa develops its own products and also has collaboration agreements with several pharma companies, among others Novo Nordisk and AstraZeneca.
Nanexa's share is listed on Nasdaq First North Growth Market in Stockholm (NANEXA).
This information is information that Nanexa is obliged to make public pursuant to the EU Market Abuse Regulation. The information was submitted for publication, through the agency of the contact persons set out above, at 2025-02-19 08:00 CET.