AMSTERDAM, Feb. 20, 2025 (GLOBE NEWSWIRE) -- New research by Teva Pharmaceuticals Europe shows that 46% of generic medicines from the EU Critical List of Medicines are supplied by only one provider. This number almost doubles to 83% of critical generic medicines when looking at suppliers with over 60% market share. The analysis, Generic Health Check Europe 3.0, shows consolidation of critical generic medicines that is 3 times faster than other generic medicines. This concentration is an actual material risk to the security of critical medicines supply in the region.
A diversified supply of critical generic medicines from multiple manufacturers is required to ensure that healthcare systems can meet the needs of their patients. The research shows that for most critical generic medicines' supply (e.g. cardiology, oncology, mental health, antibiotics), this is not the case. A worrying level of supplier consolidation and critical generic medicines product withdrawal is adding to the uncertainty. This trend has been seen in recent years, notably due to geopolitical tensions, economic challenges and new policy requirements. This puts patient care at significant risk.
Although the price of consumer goods has increased by 30% in the last 10 years, the average prices of generic prescription medicines have fallen by nearly 8%. The absence of price flexibility together with increasing regulatory and environmental requirements is impacting the economic viability of critical generic medicines, forcing suppliers to withdraw medicines from the market and limit investments in manufacturing capacity building.
Michal Nitka, Senior Vice President, Generics Head Europe & OTC Global Head at Teva, says: "Patients depend on reliable access to affordable, high-quality treatments, but the ongoing consolidation of suppliers and withdrawal of critical medicines threatens this access. Addressing the pressures on generic medicine manufacturers is essential to protecting patient care and ensuring the long-term sustainability of Europe's healthcare systems. For critical generic medicines it is even more important that a reliable, diversified supply network is in place."
Teva is calling for the following actions to be implemented as a priority:
- Developing at full scale the European Solidarity Mechanism to allow reallocation of existing market stocks to better address national shortages.
- Safeguard the economic viability of critical generic medicines by ensuring the systematic use of multiwinner, multicriteria procurement schemes that are designed in collaboration with the industry, moving away from the lowest cost purchasing bid, to recognize the most valuable purchasing offer for the European healthcare system and economy.
- Strengthening European critical medicines manufacturing competitiveness and capacity via agile funding schemes to fast-track approval for strategic investments, supporting all types of innovations including in manufacturing.
Contact:
Fiona Cohen, Teva Corporate Communications: +31 6 2008 2545
Notes to Editors:
About Teva Pharmaceuticals
Teva Pharmaceutical Industries Ltd. is a global pharmaceutical leader, harnessing our generics expertise and stepping up innovation to continue the momentum behind the discovery, delivery and expanded development of modern medicine. For over 120 years, Teva's commitment to bettering health has never wavered. Today, the company's global network of capabilities enables its 37,000 employees across 57 markets to push the boundaries of scientific innovation and deliver quality medicines to help improve health outcomes of millions of patients every day. To learn more about how Teva is all in for better health, visit www.tevapharm.com.
Appendix - key information from Teva's Generics Health Check 3.0:
What are generic medicines?
Generic medicines are manufactured once the patent of the innovator has expired. Generic medicines provide the same quality, safety and efficacy as the innovator brand product, at offering cost-effective alternative for patients and healthcare systems. 67% of prescriptions of dispensed medicines in Europe are generic)
What are critical generic medicines?
The EU list of critical medicines identifies medicines for human use for which continuity of supply in the EU is a priority. This means that for these medicines shortages should be avoided, as they are critical for EU healthcare systems to function properly. A critical medicine is identified by combining two criteria: the seriousness of the disease it targets and the availability of suitable alternative medicines.) Generics medicines on that list are considered critical generic medicines.
What is the EU Critical Medicines List?
The EU's Critical Medicines List, formally known as the Union List of Critical Medicines, identifies medicines that are essential for healthcare systems across the EU/EEA. These medicines are prioritised to ensure their continuous supply and to prevent shortages.
What are the key findings of the report?
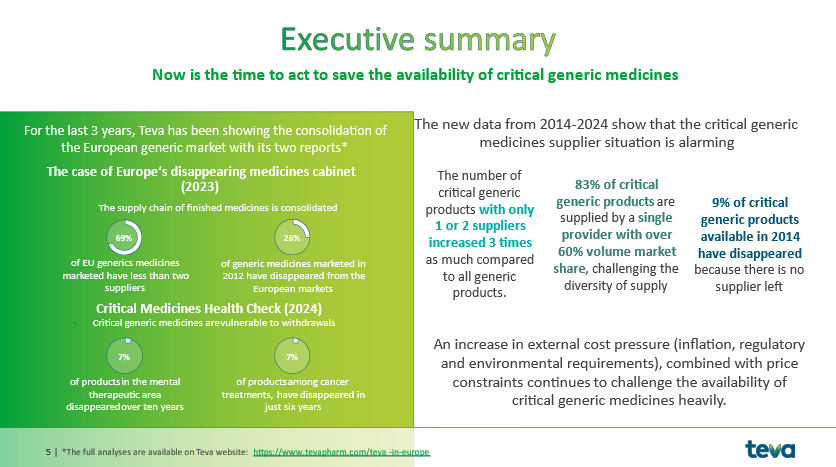
Between 2014 and 2024, the number of critical generic products with only 1 or 2 suppliers increased 3 times as much compared to all generic products.
- 83% of critical generic products are supplied by a provider with over 60% volume market share, challenging the diversity of supply
- 30% of the critical generics marketed in 2014 are no longer available in 2024
How quickly are critical generic medicines disappearing?
The proportion of critical generic medicines with just 1 or 2 makers rose by 15% while that proportion increased by 5% in other generics). In addition, the research showed that 30% of critical generic medicines marketed across the region have been withdrawn. This rises to 37% in oncology and cardiology and 33% for antibiotics.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/77122d56-c7e3-480d-bd27-5145f97f677e