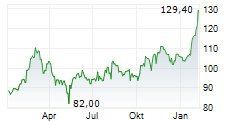
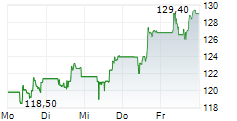
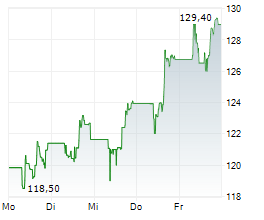
FOSTER CITY (dpa-AFX) - Gilead Sciences Inc. (GILD) announced that the European Commission has granted conditional marketing authorization for seladelpar for the treatment of primary biliary cholangitis in combination with ursodeoxycholic acid (UDCA) in adults who have an inadequate response to UDCA alone, or as monotherapy in those unable to tolerate UDCA.
The company noted that Seladelpar (an orphan designated product) is now approved and will provide an important treatment option for people living with the rare liver disease in the European Economic Area.
Primary biliary cholangitis is a rare, chronic, autoimmune disease of the bile ducts that affects approximately 22 out of 100,000 people in Europe. Primary biliary cholangitis is more common in women and causes liver damage that can progress to liver failure, particularly if left untreated.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News