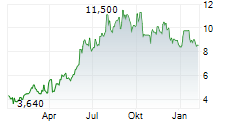
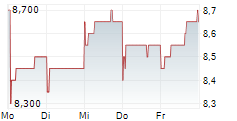
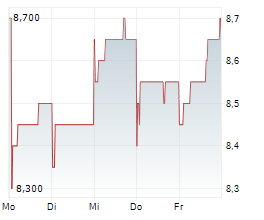
BEIJING (dpa-AFX) - Innovent Biologics Inc. (IVBXF.OB) announced that the New Drug Application or NDA for ipilimumab injection (anti-CTLA-4 monoclonal antibody) has been accepted by the Center for Drug Evaluation (CDE) of China's National Medical Products Administration (NMPA) and granted Priority Review designation in combination with sintilimab as neoadjuvant treatment for resectable microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colon cancer.
The NDA acceptance and Priority Review designation are based on results from a randomized, controlled, multicenter, pivotal Phase 3 clinical trial which evaluated the safety and efficacy of ipilimumab combined with sintilimab as neoadjuvant therapy and as compared with direct radical surgery for MSI-H/dMMR colon cancer.
The primary endpoints are pathologic complete response rate and event-free survival (EFS). Interim analysis by the Independent Data Monitoring Committee showed that the NeoShot trial has met its primary endpoint.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News