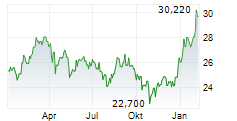
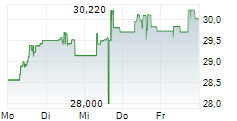
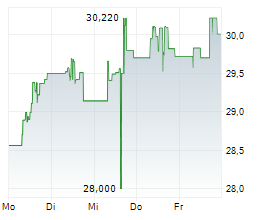
BRUSSELS/FRANKFURT/PARIS (dpa-AFX) - Takeda Pharmaceutical Co. Ltd. (TKPHF.PK, TAK) announced on Monday that the European Medicines Agency or EMA has approved an additional 2 mL pre-filled pen option for Takhzyro or Lanadelumab. This approval is for the subcutaneous administration in adolescents, aged 12 years and above, and adult patients with recurrent hereditary Angioedema.
The company said that at present, Takhzyro is approved as 150 mg solution for injection in pre-filled syringe, 300 mg solution for injection in pre-filled syringe, and 300 mg solution for injection in vial. This latest approval for an additional subcutaneous administration option, i.e, the Takhzyro 300 mg solution for injection in pre-filled pen, containing 300 mg of Lanadelumab in 2 mL of solution, has been supported with clinical studies.
Hereditary Angioedema is a rare disorder causing painful and recurring attacks of oedema or swelling in different body parts like the abdomen, face, feet, genitals, hands and throat. These painful attacks can cause asphyxiation and can be life threatening.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News