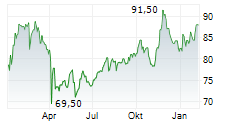
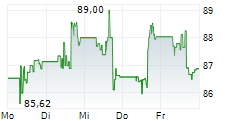
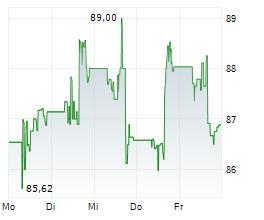
DUBLIN (dpa-AFX) - Medtronic plc (MDT), healthcare technology firm, announced Monday the U.S. Food and Drug Administration (FDA) approval of BrainSense Adaptive deep brain stimulation (aDBS) and BrainSense Electrode Identifier (EI).
Deep brain stimulation (DBS) has been transforming the lives of people with Parkinson's and other neurological disorders for more than 30 years. DBS is similar to a cardiac pacemaker, but for the brain.
DBS uses a surgically implanted neurostimulator via a minimally invasive procedure to transmit electrical signals to specific parts of the brain affected by debilitating neurological disorders.
Medtronic has now enhanced its Percept DBS neurostimulators with exclusive BrainSense Adaptive technology, introducing aDBS for people living with Parkinson's. This feature personalizes therapy based on a patient's brain activity in real time, both in clinical settings and in daily life.
It provides enhanced therapy personalization for symptom control that automatically adjusts, minimizing the need for patients to manually adjust stimulation.
The U.S. FDA approval also includes the Medtronic BrainSense Electrode Identifier (EI), which helps reduce patient time spent in clinic to program their DBS settings. By using EI, clinicians can conduct an accurate and precise initial programming, 85% faster compared to traditional electrode selection.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News