FOSTER CITY (dpa-AFX) - Gilead Sciences, Inc. (GILD) Monday announced that the European Medicines Agency has validated its applications for accelerated review of lenacapavir, a twice-yearly injectable HIV-1 capsid inhibitor for HIV prevention.
The EU-M4all application aims to fast-track lenacapavir's approval in low- and lower-middle-income countries. Phase 3 trials showed lenacapavir reduced HIV risk by 100 percent in cisgender women and 96 percent in cisgender men and gender-diverse people.
This follows the U.S. FDA's recent acceptance of lenacapavir for priority review, underscoring global interest in its potential to advance HIV prevention efforts.
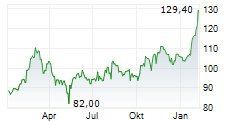
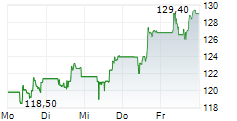
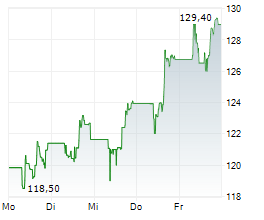
GILD is currently trading at $111.43 up 1.35 percent or $1.48 on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News