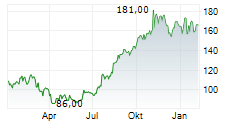
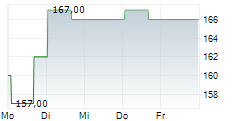
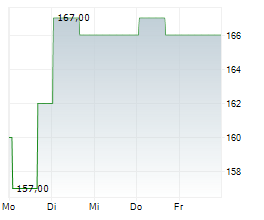
WASHINGTON (dpa-AFX) - Ligand Pharmaceuticals Inc. (LGND) Tuesday announced it has closed a royalty financing agreement with Castle Creek Biosciences, Inc. to support the Phase 3 clinical study of D-Fi (FCX-007), Castle Creek's lead candidate, in patients with dystrophic epidermolysis bullosa (DEB).
Ligand originated, structured, and invested $50 million and led a syndicate of co-investors who invested $25 million in exchange for a high-single digit royalty on worldwide sales of D-Fi. The syndicate includes existing Castle Creek investors Paragon Biosciences and Valor Equity Partners and new investor XOMA Royalty Corporation (XOMA).
'Partnering with Castle Creek is an exciting opportunity to advance an orphan drug-designated gene therapy for a serious unmet medical need through Phase 3 development,' said Todd Davis, CEO of Ligand. 'This collaboration reflects our commitment to invest in groundbreaking de-risked treatments that can transform patients' lives and expand our diversified portfolio of revenue-generating assets.'
'We are pleased Ligand and our syndicate of investors recognized the potential of this critical therapy, which we believe represents a significant step forward in addressing the needs of patients living with DEB,' commented Matthew Gantz, president and CEO of Castle Creek. 'Having a sophisticated investor like Ligand work closely with our extremely supportive equity partners made this transaction to support our Phase 3 clinical trial possible.'
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News