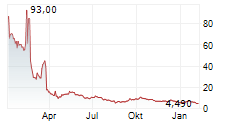
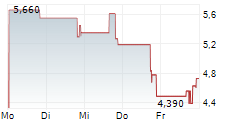
Cash on hand provides over one year of runway to fund expected operations
HOUSTON, TX / ACCESS Newswire / February 26, 2025 / CNS Pharmaceuticals, Inc. (NASDAQ:CNSP) ("CNS" or the "Company"), a biopharmaceutical company specializing in the development of novel treatments for primary and metastatic cancers in the brain and central nervous system, today provided an update on its current cash position and discussed the completion of its recent initiative to maintain its listing on the NASDAQ Capital Market ("NASDAQ").
"As we kick off 2025, the Company has never been fundamentally stronger. Our team conducted a global trial of Berubicin, and we remain on track to announce our primary data analysis in the first half of 2025," commented John Climaco, Chief Executive Officer of CNS Pharmaceuticals. "On the financial front, we now have the necessary capital to fund planned operations into the first quarter of 2026. To be precise, this means not just continuing and expanding our work based on the aforementioned Berubicin data but also charging toward our goal of commencing our TPI 287 clinical program by consenting our first patient before the end of the year. We have begun what we believe will be the most transformational year in the history of the Company and have high hopes that the GBM community and our stakeholders will be celebrating this, along with the patients impacted by this disease, in the very near term."
Mr. Climaco continued, "Additionally, maintaining our listing on Nasdaq is a top priority for the Company. Given our expected primary analysis in the first half of 2025 and our plans to advance TPI 287, we believe our listing on NASDAQ supports the strength of the company we believe will exist in the second half of 2025 following the completion of key milestones. We are executing to the best of our abilities to remain listed and will continue to do so."
As of February 26, 2025, the Company's current cash position has increased to $14 million. Based on current projections, management believes the current cash on hand is sufficient to fund operations into 2026, including the expected upcoming catalytic milestones.
As previously announced, the Company implemented a reverse stock split of the Company's common stock in order to regain compliance with NASDAQ's minimum bid price requirement.
About CNS Pharmaceuticals, Inc.
CNS Pharmaceuticals is a clinical-stage pharmaceutical company developing a pipeline of anti-cancer drug candidates for the treatment of primary and metastatic cancers of the brain and central nervous system.
The Company's lead drug candidate, Berubicin, is the first anthracycline to appear to cross the blood-brain barrier. Berubicin is the subject of the Company's late-stage, fully-enrolled, global clinical trial for the treatment of glioblastoma multiforme (GBM), an aggressive and currently incurable form of brain cancer. The Company expects to release primary analysis data on Berubicin's performance in this designed-to-be pivotal trial before the end of the first quarter of 2025.
The Company's second drug candidate, TPI 287, is an abeotaxane which stabilizes microtubules and inhibits cell division, causing apoptosis and cell death. Similar to Berubicin, TPI 287 has shown the potential to cross the blood-brain barrier and treat CNS tumors. TPI 287 has been well tolerated in over 350 patients to date, including in clinical trials as a monotherapy and in combination with bevacizumab for the treatment of recurrent neuroblastoma and medulloblastoma, as well as refractory prostate cancer and melanoma, and in tauopathy disease, which can result in dementia.
For more information, please visit www.CNSPharma.com, and connect with the Company on X, Facebook, and LinkedIn.
Forward-Looking Statements
Some of the statements in this press release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this release include, without limitation, the timing of the release of the primary analysis data on Berubicin's performance and the Company's cash runway. These statements relate to future events, future expectations, plans and prospects. Although CNS believes the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. CNS has attempted to identify forward-looking statements by terminology including 'believes,' 'estimates,' 'anticipates,' 'expects,' 'plans,' 'projects,' 'intends,' 'potential,' 'may,' 'could,' 'might,' 'will,' 'should,' 'approximately' or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including market and other conditions and those discussed under Item 1A. "Risk Factors" in CNS's most recently filed Form 10-K filed with the Securities and Exchange Commission ("SEC") and updated from time to time in its Form 10-Q filings and in its other public filings with the SEC. Any forward-looking statements contained in this press release speak only as of its date. CNS undertakes no obligation to update any forward-looking statements contained in this press release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events, except as required by law.
CONTACTS:
Investor Relations Contact
JTC Team, LLC
Jenene Thomas
908.824.0775
CNSP@jtcir.com
SOURCE: CNS Pharmaceuticals, Inc.