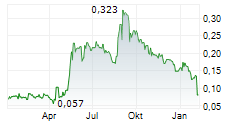
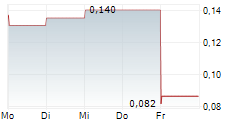
aXichem's development during the period 2024-01-01-2024-12-31
In this interim report, the aXichem group is presented, which consists of aXichem AB (publ), Incendia Pharma AB and aXichem AS (Norway). Amounts in brackets refer to the corresponding period of the previous year.
aXichem's development 2024-01-01 - 2024-12-31
Turnover and results
The group's net sales amounted to SEK 8,570 thousand (SEK 1,809 thousand).
Operating profit amounted to SEK -15,827 thousand (SEK -17,318 thousand).
The group's profit after tax amounted to SEK -17,815 thousand (SEK -20,814 thousand).
Financial net SEK -1,956 thousand (SEK -3,471 thousand).
Cash flow and financial position
The group's cash and cash equivalents on December 30, 2024 amounted to SEK 14,359 thousand (SEK 4,309 thousand).
The equity ratio amounted to 94% (79%).
Investments
Investments in intangible, tangible or financial fixed assets have during the year been with SEK 6,499 thousand (SEK 5,103 thousand).
Depreciation
Depreciation of intangible and tangible fixed assets has during the year been done with SEK 4,277 thousand (SEK 3,834 thousand).
Stock data
Profit as of December 31, 2024 corresponds after tax to a profit per share of SEK -0,46 (-1,03).
Equity per share at the end of the period amounted to SEK 1,19 (SEK 2,26).
aXichem's development 2024-10-01 - 2024-12-31
Turnover and results
Net sales amounted to SEK 1,391 thousand (SEK 275 thousand).
The operating profit amounted to SEK -4,864 thousand (SEK -4,801 thousand).
The group's profit after tax amounted to SEK -5,314 thousand (SEK -5,532 thousand).
Financial net SEK -418 thousand (SEK -706 thousand).
Investments
Investments in intangible, tangible or financial fixed assets amounted to SEK 2,764 thousand (SEK 523 thousand).
Depreciation
Depreciation of intangible and tangible fixed assets amounted to SEK 1,137 thousand (SEK 1,016 thousand).
Share data
After tax, the periods result corresponds to a result per share of SEK -0,10 (SEK -0,26).
Events during the period
First quarter
- On January 5, 2024, it was announced that aXichem's distributor Chr. Olesen has prepared the first batch of aXiphen for shipment to Chr. Olesen's facilities in Brazil from where it will be delivered to industrial scale production trials in the Brazilian market.
- On January 10, 2024, aXichem announced new orders for aXivite® from Uriach related to the agreement announced in June 2023. The orders are related to the production of a new melatonin formula for Uriach's brand Aquilea® featuring aXivite®. The first order was announced in November 2023, and with the additional orders placed, the total order value from Uriach, to date, amounts to about 60,000 Euro.
- On January 15, 2024, the company announced that a new product, GLP-Activate with aXivite, is launched by the U.S. based company Triquetra Health. The GLP-1 hormone (Glucagon-like peptide 1) is produced in the intestine and is considered to control appetite, cravings, blood sugar and most facets of metabolism. Triquetra Health's product GLP-Activate can provide the body natural extracts and nutrients that can support the body's own GLP-1 production. The GLP-1 hormone has been identified as one of the keys to healthy weight management and metabolic health.
- On January 30, 2024, aXichem announced that the company has signed a Letter of Intent (LOI) with Silvaco A/S, a key player in the nutraceutical, pharmaceutical, food, feed, and cosmetics industries in Scandinavia. This LOI marks the beginning of a promising collaboration aimed at introducing aXivite, into the Scandinavian dietary supplement market.
- On February 1, 2024, it was announced that the Board, subject to subsequent approval by the General Meeting, had resolved on a new issue of shares and warrants ("Units") with pre-emptive rights for existing shareholders of approximately SEK 40.3 million before issue costs (the "Rights Issue"). One unit consists of five shares of class A and five warrants of series TO1A. The subscription price per Unit is SEK 7.50, corresponding to SEK 1.50 per share. The warrants are issued free of charge. The Rights Issue is covered to 70 percent by subscription and guarantee commitments.
- On February 13, 2024, it was announced that the company has signed a Letter of Intent (LOI) with Silver Fern Brand, a provider of premium health supplements. This collaboration aims to integrate aXivite into a broader product range, meeting the demand for science-backed health supplements.
- On March 6, 2024, an extraordinary general meeting was held. The meeting decided in accordance with the board's proposal to amend the articles of association with regard to limits on the number of shares and share capital. The share capital must be a minimum of SEK 4,250,000 and a maximum of SEK 17,000,000. The housing company must have a minimum of 21,400,000 and a maximum of 85,600,000 shares. Furthermore, the meeting decided in accordance with the board's proposal to carry out a rights issue of Units.
- On March 13, 2024, it was announced that dietary supplement supplier OmneDiem® is launching two new products on the US market with aXichem's innovative ingredient aXivite®. These launches represent an expansion of aXivite's applications in the areas of exercise and health and are another step in the company's work to broaden its global market presence and customer base.
- On March 19, 2024, aXichem announced that the strategic launch of aXiphen in Brazil will take place at the two main industry events: Nucleovet, April 9-11, and the South American International Poultry and Swine Show (SIAVS), August 6-8. The launch is supported by a marketing campaign aimed at maximizing the product's introduction to the Brazilian market.
- On March 26, 2024, the company announced the outcome of the share issue, the "Rights Issue", whose subscription period ended on March 25, 2024. A total of 2,701,257 Units were subscribed, corresponding to approximately 50.3 percent of the Rights Issue, with the support of unit rights. In addition, 14,182 Units were subscribed without the support of unit rights, corresponding to approximately 0.3 percent of the Rights Issue. Finally, 1,046,419 Units were subscribed according to the guarantee commitments entered into, corresponding to approximately 19.5 percent of the Rights Issue. A total of 3,761,858 Units were thus subscribed and the Rights Issue was thus subscribed to 70 percent. Through the rights issue, approximately SEK 28.2 million will be injected to aXichem before issue costs. Through the Rights Issue, the number of shares in aXichem increases by 18,809,290, from 21,496,325 to 40,305,615, and the share capital increases by SEK 3,761,858, from SEK 4,299,265 to SEK 8,061,123. Upon full utilization of the subscription options of series TO1A issued in connection with the Rights Issue, the number of shares will increase by a further 18,809,290, from 40,305,615 to 59,114,905, and the share capital will increase by a further SEK 3,761,858, from 8,061 SEK 123 to SEK 11,822,981.
Second quarter
- On April 24, a new order for aXivite® was announced within the agreement with the pharmaceutical company Uriach. The order is linked to the production of a new melatonin formulation for Uriach's brand Aquilea® with aXivite®. This means that the total order value from Uriach, within the agreement the parties have, now amounts to approximately 150,000 Euro. aXichem also received an order for aXivite® from its Spanish distributor Pharmafoods worth 13,700 Euro for the development of a new consumer product to be launched later this year.
- On May 29, 2024, aXichem's annual report for 2023 was published. On June 19, the company held an annual general meeting and on the same day the communique from the general meeting was published. The minutes from the meeting can be read at https://www.axichem.com/arsstamma-2024/
- On June 3, 2024, it was announced that aXichem launched a product-specific website, www.axiphen.se, for its salmonella inhibitor, aXiphen®, targeting the Brazilian market for poultry production and poultry feed. Brazil is one of the global market leaders in the production and export of chicken meat.
- On June 25, 2024, the company announced a significant new order from its US distributor SEE Nutrition. The order, which is worth SEK 3 million, concerns one ton of aXivite®, aXichem's scientifically documented synthetic capsaicin product. SEE Nutrition markets and sells aXivite® to suppliers of nutritional products and dietary supplements for sports, fitness, pre-workout and weight control in the American market.
Third quarter
- On August 14, 2024, the company announced that it together with the distributor Chr. Olesen will conduct two commercial production tests with aXiphen in collaboration with poultry producers with a significant export business. aXiphen is now delivered from Chr. Olesen to the producers, who are both based in southern Brazil. The tests have a positive impact on aXichem's cash flow of around two million kroner as the product now moves from distributor to end customer.
- On August 27, 2024, aXichem announced that the board of directors, in its annual revision of the company's communication policy, has decided that from now on aXichem shall not publish any financial forecasts or forecast-like targets. The board's decision means that the company's total sales are presented in the quarterly reports and previously communicated sales targets will not be commented on.
Fourth quarter
- On October 7, 2024, it was announced that the subscription price for warrants of series TO1A issued as part of the rights issue completed in April 2024 has been set at SEK 0.95. The subscription price of the warrants corresponds to 70 percent of the volume-weighted average price of the company's shares on the Nasdaq First North Growth Market during the period 1 October - 7 October 2024.
- On October 16, 2024, the company announced that an agreement had been entered into for free guarantee commitments (so-called "top-down" or "top guarantee") in the ongoing redemption of warrants series TO1A. The guarantee commitments comprise a total of SEK 2.0 million, corresponding to approximately 11 percent of the issue proceeds that the Company can contribute through the redemption of TO1A.
- On October 23, 2024, the outcome of the exercise of the warrants series TO1A, which were issued in connection with the Company's preferential issue of units in March 2024, was announced. A total of 17,862,853 TO1A were exercised, corresponding to a subscription rate of approximately 95 percent, for the subscription of 17 862,853 new A shares in aXichem. aXichem will receive approximately SEK 17.0 million before issue costs from the exercise of warrants series TO1A.
- On October 24, 2024, it was announced that the board of aXichem, in line with previous communication, decided on a targeted new issue of 946,437 A shares, to the investors who provided a so-called top guarantee in connection with the exercise period regarding the warrants of series TO1A. The subscription price in the directed new issue amounted to SEK 0.95 per share, corresponding to the subscription price when using TO1A. Through the directed new issue, SEK 0.9 million is added to the Company before issue costs.
- On December 17, 2024, the company announced the launch of a new aXivite formulation, aXivite TR (Target Release). aXivite TR is specifically designed and developed to be the active ingredient in sports nutrition and weight management products, maintaining the scientifically documented positive effects of phenylcapsaicin, but completely erasing the sensation of pungency.
Events after the period's end
- On February 4, 2025, it was announced that the company will increase the number of studies, regarding effect, that form part of the documentation for supplementing aXichem's application for Feed Additive approval in the EU for the company's product phenylcapsaicin as a salmonella-inhibiting additive in chicken feed. The company will conduct one additional effect study and assesses, based on previous positive effect data, that this is the only remaining part for a complete application. The extended studies affect the estimated time of submission to the European Food Safety Authority (EFSA), which the company announced in connection with the presentation of the quarterly report on November 29, 2024.
- On February 12, 2025, it was announced that the company had received an order for its feed additive product, aXiphen, from the distributor Chr. Olesen. The order value amounts to about SEK 7 million and delivery will take place to Chr. Olesen's operations in Brazil by call-off during the current year.
CEO's statement
The operating year 2024 has ended and the year has been aXichem's strongest so far in terms of sales, with net sales for the year amounting to 8,570 thousand, which is very gratifying. Looking at the year's fourth quarter net sales amount to SEK 1,391 thousand, coming from sale of aXivite, and the operating profit of the quarter amounts to SEK -4,864 thousand. Operating expenses for the quarter amount to SEK -6,346 thousand and are primarily linked to costs for marketing and sales activities and to costs for completing the company's application for Feed Additive approval in the EU.
The launch of aXiphen in Brazil continues
We entered 2024 with a fresh approval in Brazil for aXiphen and a goal to be able to conduct tests with a number of poultry producers during the year, in collaboration with our distributor Chr. Olesen. It is clear that the need to prevent salmonella is great, as is the interest from producers. The marketing activities in the form of advertising have given the product good attention. However, it took a little longer than we expected to start testing with larger poultry producers, but the process is underway and Chr. Olesen's team, like us, is positive about the development and the potential of aXiphen. We received this confirmation in the form of the order we received from Chr. Olesen after the end of the period. The value of the order, which will be delivered according to call-off in 2025, amounts to approximately SEK 7 million. A really good start to 2025 and an important milestone for us in the launch of aXiphen.
Two positive studies on the efficacy of aXiphen completed
During the fourth quarter, we continued our work on supplementary studies for our application to the European Food Safety Authority (EFSA) for Feed Additive approval for phenylcapsaicin in the EU. After the end of the period, on February 4, 2025, we announced that we had conducted a total of three efficacy studies with aXiphen, two of which show significant data regarding positive effects as a salmonella inhibitor, but that one study did not fully provide the results we desire in order to be able to supplement the application. EFSA requires three studies with significant positive data and we therefore decided to expand with another study. Our assessment is that when it is completed, we will have the final part in place for a complete application. We will notify the market when there is any significant change in the process and when submission is made.
aXivite had its best year yet in 2024
In the dietary supplements business area, aXivite has gradually shown its potential during the year. The training and weight control segments are strongly characterized by various trends and the US market is leading the way. In the US, we continue our collaboration with SEE Nutrition, which during the year gave us sales of three million SEK. Through SEE Nutrition, we continuously receive various ideas from producers who want to utilize the effect of phenylcapsaicin. Some of these ideas fit like a glove for our ingredient, one such example is the Silver Fern Brand, which has received undividedly positive reviews for its product "Metabolism", which contains aXivite, among other things. In order to be able to satisfy more exciting requests for end products, we launched a new formulation of aXivite, aXivite® TR (Target Release), in the fourth quarter. aXivite® TR is specially designed and developed to be the active ingredient in dietary supplements in the sports, training and weight control segment, where the heat of the product is not felt, but where the effect of phenylcapsaicin is the same.
Our agreement with the pharmaceutical company Uriach, which gives Uriach exclusivity for a melatonin formulation with aXivite, has contributed to our sales in 2024 in an excellent way. This means that the commitment within the framework of the agreement, which included 400,000 Euros has been well fulfilled. Uriach has launched its melatonin product, Aquilea - with aXivite as a bio-enhancer, in Spain in 2024. The plan is now for Portugal, Germany, Austria and Romania to follow and it will be exciting to follow the development.
With strengthened cash position into a new financial year
I would like to take this opportunity to thank the shareholders for the trust they have shown in the company during the year, not least in connection with the rights issue of units that was carried out in March 2024 and the exercise of the options, TO1A, in October. This provided the company with a total of SEK 46 million before issue costs during the year. With this, we have been able to continue to drive the business forward, carry out important studies for the Feed Additive application and activity for production, market and sales. It also feels satisfying that the company is now debt-free after repayment of the convertible loan, equivalent to approximately SEK five million, issued by Formue Nord.
Together with aXichem's team and board, I look forward to continuing the work on the commercialization of phenylcapsaicin as an ingredient for dietary supplements and additives in animal feed. I plan to be in Stockholm at the Aktiespararna event Stora Aktiedagen on March 12, 2025, at 2:20 PM. I hope that those of you who have the opportunity will listen in. Please also follow aXichem on LinkedIn where we regularly post about the company's activities.
Torsten Helsing, CEO
About aXichem AB (publ)
aXichem's business concept is to develop, patent and market nature-analog industrial compounds. aXichem's product is a natural analog substance called phenylcapsaicin, which is a synthetically produced and patented capsaicin. As phenylcapsaicin is synthetically produced and has several advantages compared to natural capsaicin, such as controllable quality and production process.
The product has benefits that makes it commercially interesting in several application areas, for example as ingredient in animal feed, as ingredient in dietary supplements, as a bio-enhancer and as a bio-repellent in marine applications. aXichem is currently marketing the product under different brands in two prioritized areas: aXivite® for dietary supplements and aXiphen® for poultry feed. aXiphen® has shown properties as a growth promoter and anti-salmonella additive in studies made in commercial poultry production settings. In the market segment for sports nutrition products aXivite® is today included in several products for weight control, improved metabolism, and gut health.
aXichem aims to become a global supplier of industrial natural analogue chemicals to players in the chemical industry who manufacture products containing aXichem's raw materials. The company's share is listed on NASDAQ First North since 2013.
Future prospects
aXichem's commercialization of phenylcapsaicin under the brands aXiphen® in the animal feed market and aXivite® in the food supplement market continues. The production capacity and logistics through subcontractors are in place, as is a production process optimized for commercial volumes. The pace of development in the establishment of the products is determined by market approvals in the strategically important countries or regions, starting with the EU, USA, Brazil and India. aXichem cooperates with specialized distributors, who either have their own production or cooperate with innovative leading producers.
Within the Dietary Supplements business area, the company has market approval for aXivite® in the EU and in North America, and market establishment of the company's product is ongoing.
Within the Animal Feed Business Area, aXiphen® has been approved in Brazil since December 2023 for sale as an ingredient in chicken and pig feed, and market establishment is in an early phase. An application for market approval for aXiphen® in the EU is being processed by regulatory authorities and a corresponding application for the US market is being prepared. The company's strategic focus is to prioritize Brazil and the EU, as these markets have the best conditions for an initial establishment.
Financing
February 1, 2024, it was announced that the company's board, subject to the approval of the general meeting, had decided to carry out an issue of shares and warrants ("Units") with preferential rights for existing shareholders ("Rights Issue") of approximately SEK 40.3 million before issue costs. A Unit consisted of five A shares and five warrants of series TO1A. The subscription price per Unit amounted to SEK 7.50, corresponding to SEK 1.50 per A share. The warrants were issued free of charge. The rights issue was covered to 70 percent by subscription and guarantee commitments. The boards decision was approved at an extraordinary general meeting, which was held on March 6, 2024.
The board also decided to take out a new convertible loan from Formue Nord Fokus A/S of approximately SEK 5.3 million, which partially replaced the existing convertible loan.
The outcome of the rights issue, which was 70 percent subscribed, was announced on March 26, 2024. Through the rights issue, approximately SEK 28.2 million was added to aXichem before issue costs. The number of shares in aXichem increased by 18,809,290, to 40,305,615, and the share capital increased by SEK 3,761,858, to SEK 8,061,123.
During the period 1-7 October, warrants of series TO1A were exercised at a rate of SEK 0.95. The warrants' subscription price corresponded to 70 percent of the volume-weighted average price for the company's shares on the Nasdaq First North Growth Market. The outcome from the exercise of the warrants series TO1A amounted to 17,862,853 shares. In addition, a targeted new issue of 946,437 A shares was carried out, to the investors who provided a so-called top guarantee in connection with the exercise period regarding the warrants of series TO1A. This meant that the company received SEK 18,809,290 before transaction costs and that the number of shares increased by 18,809,290, from 40,305,615 to 59,114,905, and that the share capital increased by a further SEK 3,761,858, from SEK 8,061,123 to 11,822,981 SEK.
Incentive program - Employee stock options
At the annual general meeting on May 31, 2022, it was decided on an option program of series 2022/2026 for employees and key persons in the company comprising 400,000 options with the right to subscribe for 400,000 A shares. As of the balance sheet date, 270,000 options were allocated to staff and key persons, of which 101,250 were vested.
The employee options are earned over 4 years, with a quarter each year, provided that the participant is employed by or otherwise engaged in the company on the grant date. The staff options are awarded free of charge. Earned employee options can be exercised during a three-year period, however no earlier than three years after the respective grant date. Each employee option gives the right to subscribe for 1 A share at a subscription price that corresponds to 140 percent of the volume-weighted average price for the company's A share during the five trading days immediately preceding the day on which the employee options are awarded. The subscription price and the number of A shares to which each employee option entitles may be subject to recalculation as a result of a bonus issue, split, issues or similar measures. In order to enable the delivery of shares according to the incentive program, it was also decided to issue a maximum of 400,000 warrants.
Ownership structure
For information about the company's owners, see the company's website www.axichem.com under https://www.axichem.com/investor-relations/investor-structure
For information on insider trading, see the Financial Supervisory Authority's register.
Certified Adviser
Västra Hamnen Corporate Finance is aXichem's Certified Adviser.
Number of shares
The number of shares and votes in the company as of 31 December 2024 was 59,114,905 and the company's share capital amounted to 11,822,981 SEK SEK. The company has only one class of shares, shares of series A, with 1 vote per share. The quota value is 0.20 SEK per share.
Accounting principles
The company applies the Annual Accounts Act and BFNAR 2012:1 Annual accounts and consolidated accounts (K3) when preparing its financial reports. The accounting principles are unchanged compared to the most recently submitted annual report.
Dilution per share
aXichem has outstanding employee options and convertible debt. There is no dilutive effect on earnings per share as long as the group's earnings are negative.
Convertible debt
Convertible liabilities are reported divided into a debt part and an equity part. The fair value of the debt part at the time of issue is calculated by discounting the future payment flows with the current market interest rate for a similar debt, without the right to conversion. The value of the part reported in equity is calculated as the difference between the issue proceeds and the fair value of the financial debt. The part reported in equity consists of the value of the built-in option to convert the debt instrument into shares. The interest expense is reported in the income statement and is calculated according to the effective interest method.
Loan expenses
Loan expenses are charged to the result for the year to which they relate.
Definition of key figures
Solidity
Adjusted equity as a percentage of total assets.
Earnings per share
Profit for the year divided by the average number of shares.
Equity per share
Equity divided by the number of shares in the market at the end of the year.
Personnel
The group had as of 31 December 2024 seven employees.
Significant risks and uncertainties
Regulatory issues are considered to be the single largest risk for the company. The effects of Covid-19 still have some impact on the company's operations in Asia. The changed security situation in Europe and the tragic development in Ukraine do not currently affect aXichem's operations, but we are following the development closely in order to be able to manage any possible effects.
Review
This interim report has not been reviewed by the company's auditor.
Financial calendar
Year End Report 2024 2025-02-27
Q1 Report, Jan - March 2025 2025-05-22
Annual Report 2024 2025-05-28
Annual General Meeting 2025-06-18
Q2 Report, Jan - June 2025 2025-08-28
Q3 Report, Jan - Sept 2025 2025-11-28
Year End Report 2025 2026-02-26
The Board of Directors and the CEO assures that the interim report provides a fair overview of the company's operations, position and results, and describes the significant risks and uncertainties that the company faces.
Lund, 27 February 2025
The Board of Directors of aXichem AB (publ)
The information was submitted, through the care of the contact person below, for publication on 27 February 2025, at 08:30 AM CET.
The report is published on the company's website under Investor Relations. Direct link to the report: https://www.axichem.com/investors-relations/financial-reports
Company Contact
Torsten Helsing, CEO
T. +46 70 686 33 55, E. torsten.helsing@axichem.se