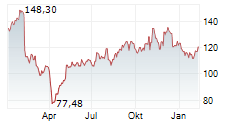
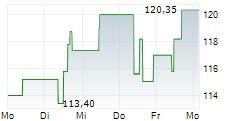
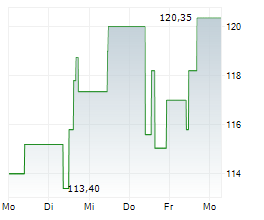
WASHINGTON (dpa-AFX) - Neurocrine Biosciences Inc. (NBIX) announced top-line data from a Phase 4 study, KINECT-PRO, demonstrating clinically meaningful and sustained effects of INGREZZA (valbenazine) capsules on the physical, social and emotional impacts experienced by patients living with tardive dyskinesia, irrespective of tardive dyskinesia severity or underlying psychiatric condition.
The company noted that the KINECT-PRO is the first study to show patient-reported impact of a vesicular monoamine transporter 2 inhibitor, specifically INGREZZA, on tardive dyskinesia or TD using multiple clinically validated scales, including the Tardive Dyskinesia Impact Scale used to evaluate the physical, social and emotional impact of involuntary movements.
The company noted that the patient-reported outcome measures provide a more complete perspective on a patient's experience of living with TD and the broad range of improvements that occurred following treatment with INGREZZA.
According to the company, results showed significant and sustained improvements from baseline in all three patient-reported outcome measures, including patients with either mild or moderate/severe tardive dyskinesia, with improvements observed as early as four weeks at the lowest dose (40 mg). AIMS scores also showed sustained reductions in involuntary movements, regardless of TD severity or underlying psychiatric condition. In the study, safety and tolerability of treatment was consistent with the known profile of INGREZZA, with no new concerns identified.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News