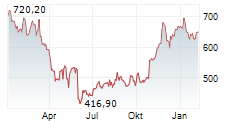
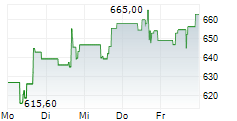
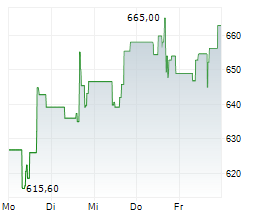
BRUSSELS/FRANKFURT/PARIS (dpa-AFX) - Regeneron Pharmaceuticals (REGN) announced the CHMP has adopted a positive opinion recommending conditional marketing authorization of linvoseltamab to treat adults with relapsed and refractory multiple myeloma. The European Commission is expected to announce a final decision in the coming months.
The company noted that the recommendation is specific to those who have received at least three prior therapies, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody, and have demonstrated disease progression on the last therapy.
Earlier in the current month, the FDA accepted for review the Biologics License Application for linvoseltamab. The target action date for the FDA decision is July 10, 2025.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News