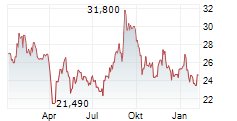
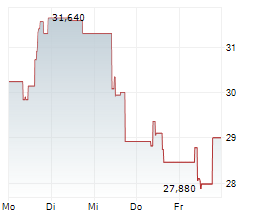
WESTON (dpa-AFX) - Japanese pharmaceutical company Eisai Co. Ltd. (ESALF.PK), a partner of BioArctic AB (publ) (BRCTF.PK), Friday announced that the Committee for Medicinal Products for Human Use or CHMP of the European Medicines Agency has reaffirmed its positive opinion for lecanemab for the treatment of early Alzheimer's disease.
Following this, the European Commission will resume the decision-making process for lecanemab's marketing authorisation.
Earlier this year, the Commission has asked CHMP to re-evaluate its information on safety of lecanemab, and check whether it requires an update to its previous opinion about the product.
If the Commission approves the marketing authorization application, lecanemab would be available in all 27 European Union member states, as well as Iceland, Liechtenstein, and Norway.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News