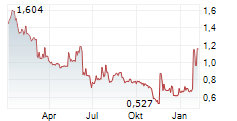
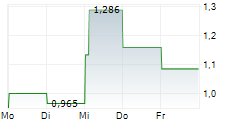
DJ VALBIOTIS SA: Valbiotis unveils its annual financial statements and confirms its strategic and financial goals
VALBIOTIS SA
VALBIOTIS SA: Valbiotis unveils its annual financial statements and confirms its strategic and financial goals
03-March-2025 / 17:40 CET/CEST
Dissemination of a French Regulatory News, transmitted by EQS Group.
The issuer is solely responsible for the content of this announcement.
=----------------------------------------------------------------------------------------------------------------------
Valbiotis unveils its annual financial statements
and confirms its strategic and financial goals
-- A key fiscal year marked by the Company's comprehensive transformation as it prepared for its
commercialization phase and first market launch
-- After the successful launch of Valbiotis®PRO Cholesterol in France, the roll-out of Valbiotis®PRO
Metabolic Health has been on schedule since its launch on February 3rd, and the launch of TOTUM.854 is still on
track for the second quarter of 2025
-- Cash position at the end of 2024: EUR11.6M, not accounting for the 2023 RTC [CIR] (EUR1.6M), which is still
being processed
-- Confirmation of all the financial goals unveiled as part of the roadmap announced last January:
2027: Turnover in excess of EUR25M, backed by positive EBITDA in France. Two targets that may be revised upwards based on
the income generated by international partnerships.
2030: Break EUR100M in turnover, with at least 30% from international sales, and an EBITDA margin of between 25 and 30%.
La Rochelle, March 3, 2025 (5:40 PM CEST) - Valbiotis (FR0013254851 - ALVAL, PEA / PME eligible), a French scientific
research laboratory specialized in the marketing of dietary supplements scientifically proven to prevent metabolic and
cardiovascular diseases and maintain optimal daily health, announces its results for fiscal year 2024 and confirms its
strategic and financial roadmap to 2030.
Milestones Reached in 2024 and Early 2025
The transformation takes shape with the success of the Company's 1st market launch
In 2024, Valbiotis transformed itself from an R&D company into a commercial enterprise fully focused on generating
sales, backed by clinically tested natural health supplements. This development led to the successful launch of
Valbiotis®PRO Cholesterol (based on the active ingredient Lipidrive®) in France last May. Following the end of the
partnership with Nestlé Health Science in June, Valbiotis has accelerated its structuring and the resizing of its
organization. By the end of 2024, 50% of the workforce was dedicated to sales, marketing and communication (vs. 11% at
the end of 2023), including a sales force of 16 Medical Promotion Officers (MPO).
Since last November, Valbiotis®PRO Cholestérol has been listed with almost all wholesaler-distributors (EDI connection)
covering 19,000 pharmacies. In the fourth quarter of 2024, 943 orders for Valbiotis®PRO Cholestérol were recorded in
pharmacies and on the web (+49% compared with the previous quarter), in line with high rates of restocking in
pharmacies (48% in December 2024) and re-purchasing on the internet (45%). Since the beginning of the year, the
Company's trajectory remains perfectly in line with its business plan.
This first market launch in the Valbiotis®PRO range was followed by that of Valbiotis®PRO Metabolic Health (based on
TOTUM.63) on February 3rd. This second product has also been very well received by healthcare professionals and
patients alike.
Clinical development now complete on 3 products in the portfolio
In fiscal year 2024 and the first few weeks of 2025, two active substances in the Valbiotis portfolio completed their
clinical trials:
- TOTUM.854, with a Phase II/III INSIGHT study positioning it as a highly promising non-drug solution for
reducing systolic blood pressure (October 2024).
- Lipridrive? (marketed under the brand name Valbiotis®PRO Cholesterol), whose Phase II/III HEART II study
confirmed its efficacy in reducing LDL Cholesterol ("bad cholesterol") in individuals with mild to moderate
hypercholesterolemia (January 2025).
TOTUM.63 (marketed under Valbiotis®PRO Metabolic Health), successfully completed its clinical development at the end of
2023, meaning the Company's portfolio is now supported by three products with unequivocal proof of efficacy. The fourth
active substance in the portfolio, TOTUM.448, is supported by an innovative research chair in hepatic steatosis, in
partnership with Laval University (Quebec).
Strengthened governance to support the Company's transformation
Supported by perfectly sized teams and experienced management, Valbiotis' transformation is being carried out under the
guidance of a fully mobilized Board of Directors, which has recently been strengthened by the arrival of two new
members:
- Stanislas Sordet, Chief Financial Officer since last spring, with financial expertise forged in both
large groups (Laboratoires Urgo, Sanofi-pasteur-MSD) and smaller organizations, joined the Board of Directors in
July 2024.
- Sébastien Poncet, current Business Unit Director for France, with over 20 years' experience in the
dietary supplement industry (Omega-Pharma, Menarini, Mayoly, etc.), joined the Board of Directors in January 2025.
The five-member body also includes Sébastien Peltier (CEO and Co-Founder), Pascal Sirvent (Chief Scientific Officer)
and Murielle Cazaubiel (Chief Regulatory & Industrial Affairs and Operational Performance Officer).
2024 Financial Statements
The Company's annual financial statements, prepared in accordance with IFRS, were approved by the Board of Directors on
February 28, 2025. They were examined by an External Auditor and are available on the Valbiotis website:
www.valbiotis.com (investors section).
A controlled cost structure aligned with a strategic pivot
31/12/2024
31/12/2023
IFRS in thousands of euros IFRS
IFRS
Operating Income
Turnover 175 4,733
Other Income 4,468 2,076
Total Income 4,644 6,809
Sales Costs (2,340) (2,044)
Research & Development (4,638) (7,150)
Sales & Marketing (4,360) (2,016)
Overheads (A) (3,101) (2,161)
Share-Based Payment Expenses (A) (631) (598)
Other Operating Income
Other Operating Expenses 2 (20)
Operating Profit for the Period (10,423) (7,180)
Operating profit (10,423) (7,180)
Gross Cost of Debt (230) (188)
Other Financial Products 662
Earnings Before Tax (9,991) (7,368)
Net Income (10,025) (7,368)
Items that will not subsequently be reclassified as income
Items that will subsequently be reclassified as income
Total Income (10,025) (7,368)
In 2024, Valbiotis generated turnover of EUR175,000, entirely from the start of its own marketing activities, compared with EUR4,733,000 in 2023, which included 4.25M Swiss Francs in lump-sum payments from Nestlé Health Science - following the success of the REVERSE-IT clinical trial.
Other operating income amounted to EUR4,468,000 last year, reflecting the IFRS restatement of the balance of the upfront payment for the NHS partnership of EUR3,514,000, and the research tax credit of EUR792,000.
Expenses incurred in fiscal year 2024 totaled EUR15,067,000, compared with EUR13,989,000 a year earlier.
Sales costs rose by 14.5% to EUR2,340,000, reflecting the emphasis placed on industrialization since the start of marketing. R&D expenditure was down 35.1% (to EUR4,638,000), reflecting the completion of major clinical trials and a significant drop in personnel costs. This development has enabled Valbiotis to step up its sales and marketing efforts, whose costs have more than doubled to EUR4,360,000, with the increase in personnel costs linked to the recruitment of a sales force and sales functions. Lastly, overheads came to EUR3,101,000 in 2024, compared with EUR2,161,000 a year earlier, mainly due to the introduction of a new ERP system as part of the Company's commercialization.
In total, in 2024, the breakdown of operating expenses (excluding share-based payment expenses) is as follows: 32% in R&D, 30% in sales and marketing, 22% in overheads and 16% in sales costs.
Net income will show a loss of EUR10,025,000 in 2024, compared with a loss of EUR7,368,000 in 2023.
Cash position of EUR11.6M at the end of 2024
31/12/2024
31/12/2023
In thousands of euros IFRS
IFRS
Cash Flow from Operating Activities (11,537) (8,056)
Cash Flow from Investment Activities (61) (250)
Cash Flow from Financing Activities (1,839) 12,494
CHANGE IN CASH POSITION (13,437) 4,189
CLOSING CASH POSITION 11,580 25,017
Cash flow from operating activities will be -EUR11,537,000 in 2024, reflecting the continuing, albeit lower, level of R&D expenditure, as well as the increase in sales and marketing costs to support the start of commercialization.
Cash flow for financing activities was negative at -EUR1,839,000, mainly due to loan repayments (for EUR1,352,000).
(MORE TO FOLLOW) Dow Jones Newswires
March 03, 2025 11:41 ET (16:41 GMT)