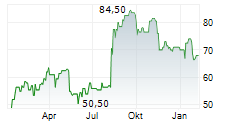
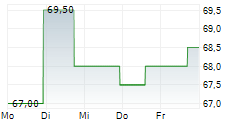
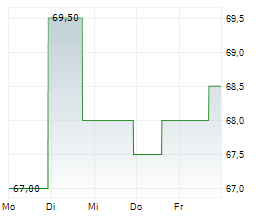
WASHINGTON (dpa-AFX) - ANI Pharmaceuticals Inc. (ANIP) announced that the U.S. Food and Drug Administration approved Purified Cortrophin Gel (repository corticotropin injection USP) (Cortrophin Gel) in a prefilled syringe format. The new presentation will be available in 40 USP units/0.5 mL and 80 USP units/mL single-dose options through Cortrophin Gels established specialty pharmacy network.
The prefilled syringe reduces administration steps for patients using Cortrophin Gel, which remains available in 5 mL and 1 mL vials.
The company noted that the new prefilled syringe reduces the steps required for patients to administer Cortrophin Gel treatment..
The company looks forward to making the Cortrophin Gel prefilled syringe available in the second quarter of 2025 as it continues to advance our Rare Disease portfolio.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News