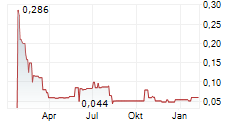
Calgary, Alberta--(Newsfile Corp. - March 5, 2025) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0) ("Hemostemix" or "HEM"), an autologous angiogenic stem cell therapy company that has safely treated 498 patients for various forms of cardiovascular disease, including peripheral arterial disease, chronic limb threatening ischemia, non ischemic dilated cardiomyopathy, ischemic cardiomyopathy, angina, congestive heart failure and vascular dementia, announces the sale of 15 ACP-01 Therapy Convertible Debentures for proceeds of USD $517,230, subject to the approval of the TSXV Exchange.
"Year-to-date, this sums to forward sales of CAD $1,149,983, which is gratifying, as it generates non-dilutive working capital for our company and its shareholders," stated Thomas Smeenk, CEO. "Importantly, forward sales enable the scheduling of both production and physician time in a very orderly manner. More over, forward sales generate a ramp-up of production from 20 to 40 treatments per month, and from 40 to 80 treatments per month, from revenue. Given 20 treatments per month generate annual revenue of USD $8,880,000 (CAD $12,876,000); given one physician completing four (4) blood draws per day generates 80 treatments per month (4 x USD $8,880,000); shareholders can quickly understand how we scale operations to our run rate as we announce additional forward sales and agreements with clinics and clinicians, globally," Smeenk stated.
An unsecured obligation of Hemostemix Inc., each ACP-01 therapy convertible debenture ("TCD") is convertible into an ACP-01 therapy on a first purchased basis. Transferable, saleable subject to a Hemostemix ROFR, will-able, and convertible into equity at the option of the Purchaser, each TCD collects interest at 6% per annum payable annually in shares of Hemostemix at the average closing price per share for the ten days preceding December 31st of each year. TCDs may be listed to trade on a stock exchange at a date in the future.
ABOUT HEMOSTEMIX
Hemostemix is an autologous stem cell therapy company, founded in 2003. A winner of the World Economic Forum Technology Pioneer Award, the Company has developed, patented, is scaling and selling autologous (patient's own) blood-based stem cell therapies that include angiogenic cell precursors (ACP-01), and later neural cell precursors (NCP-01), and cardiomyocyte cell precursors (CCP-01). Hemostemix has treated 498 patients, completed seven clinical studies of 318 subjects and published its results in nine peer reviewed publications. ACP-01 is safe, clinically relevant and statistically significant treatment for peripheral arterial disease, chronic limb threatening ischemia, non ischemic dilated cardiomyopathy, ischemic cardiomyopathy, congestive heart failure, and angina. Hemostemix completed its Phase II clinical trial for chronic limb threatening ischemia. For more information, please visit www.hemostemix.com.
For further information, please contact: Thomas Smeenk, President, CEO & Co-Founder
EM: tsmeenk@hemostemix.com PH: 905-580-4170
Neither the TSX Venture Exchange nor its Regulation Service Provider (as that term is defined under the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Information: This news release contains "forward-looking information" within the meaning of applicable Canadian securities legislation. All statements, other than statements of historical fact, included herein are forward-looking information. In particular, this news release contains forward-looking information in relation to: the forward sales of ACP-01 including the commercialization of ACP-01. There can be no assurance that such forward-looking information will prove to be accurate. Actual results and future events could differ materially from those anticipated in such forward-looking information. This forward-looking information reflects Hemostemix's current beliefs and is based on information currently available to Hemostemix and on assumptions Hemostemix believes are reasonable. These assumptions include, but are not limited to: the underlying value of Hemostemix and its Common Shares; the results of ACP-01 research, trials, studies and analyses, including the analysis being equivalent to or better than previous research, trials or studies; the receipt of all required regulatory approvals for research, trials or studies; the level of activity, market acceptance and market trends in the healthcare sector; the economy generally; consumer interest in Hemostemix's services and products; competition and Hemostemix's competitive advantages; and Hemostemix obtaining satisfactory financing to fund Hemostemix's operations including any research, trials or studies, and any Litigation. Forward-looking information is Subject to known and unknown risks, uncertainties and other factors that may cause the actual results, level of activity, performance or achievements of Hemostemix to be materially different from those expressed or implied by such forward-looking information. Such risks and other factors may include, but are not limited to: the ability of Hemostemix to complete clinical trials, complete a satisfactory analyses and file the results of such analyses to gain regulatory approval of a phase II or phase III clinical trial of ACP-01; general business, economic, competitive, political and social uncertainties; general capital market conditions and market prices for securities; delay or failure to receive board or regulatory approvals; the actual results of future operations including the actual results of future research, trials or studies; competition; changes in legislation affecting Hemostemix; the timing and availability of external financing on acceptable terms; long-term capital requirements and future developments in Hemostemix's markets and the markets in which it expects to compete; lack of qualified, skilled labour or loss of key individuals; and risks related to the COVID-19 pandemic including various recommendations, orders and measures of governmental authorities to try to limit the pandemic, including travel restrictions, border closures, non-essential business closures service disruptions, quarantines, self-isolations, shelters-in-place and social distancing, disruptions to markets, disruptions to economic activity and financings, disruptions to supply chains and sales channels, and a deterioration of general economic conditions including a possible national or global recession or depression;the potential impact that the COVID-19 pandemic may have on Hemostemix which may include a decreased demand for the services that Hemostemix offers; and a deterioration of financial markets that could limit Hemostemix's ability to obtain external financing. A description of additional risk factors that may cause actual results to differ materially from forward-looking information can be found in Hemostemix's disclosure documents on the SEDAR website at www.sedar.com. Although Hemostemix has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking information, there may be other factors that cause results not to be as anticipated, estimated or intended. Readers are cautioned that the foregoing list of factors is not exhaustive. Readers are further cautioned not to place undue reliance on forward-looking information as there can be no assurance that the plans, intentions or expectations upon which they are placed will occur. Forward-looking information contained in this news release is expressly qualified by this cautionary statement. The forward-looking information contained in this news release represents the expectations of Hemostemix as of the date of this news release and, accordingly, it is Subject to change after such date. However, Hemostemix expressly disclaims any intention or obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as expressly required by applicable securities law.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/243378
SOURCE: Hemostemix Inc.