Nightingale Health Group's Half-year report 1 July 2024 - 31 December 2024 (unaudited)
Nightingale Health's half-year report: International expansion continues in Southeast Asia and the United States
Company release, 6 March 2025 at 9:00 a.m. (EET)
This release is a summary of Nightingale Health Plc's Half-year report from period 1 July 2024 - 31 December 2024. The full Half-year report is attached to this release.
Numbers presented in brackets refer to corresponding year-on-year period unless otherwise stated.
July-December 2024 key financials (IFRS)
- Revenue was EUR 2.31 (1.71) million
- EBITDA was EUR -4.72 (-5.34) million
- Operating loss was EUR -9.13 (-9.31) million
- Net loss for the period was EUR -8.20 (-8.53) million
- Unadjusted earnings per share (EPS) was EUR -0.14 (-0.14)
- Net cash on 31 December 2024 was EUR 58.01 (30 Jun 2024: EUR 63.40) million
Significant events during the half-year period
- Nightingale Health received the second and third regulatory approvals for healthcare use from Singapore's Health Sciences Authority (HSA), the national body that regulates medical devices, therapeutics, and other healthcare products. The first approval received earlier in 2024 covered the analysis of eight common clinically used biomarkers: total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, apolipoprotein A1, apolipoprotein B, glucose, and creatinine. The second approval covered the analysis of the following fatty acids: total fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, saturated fatty acids, omega-3 fatty acids and omega-6 fatty acids. The third regulatory approval covered the analysis of alanine, glycine, histidine, isoleucine, phenylalanine, tyrosine, valine, total branched-chain amino acids, albumin, and lactate. The approval from HSA is based on the review of conformity with the safety and performance requirements set for medical devices. The HSA approval means that Nightingale Health's product satisfies the strict requirements for quality, safety, and efficacy.
- In addition, Nightingale Health's Singaporean subsidiary Nightingale Health Asia Pte. Ltd. was granted ISO 13485:2016 certification. ISO 13485 is the international quality management system standard for the design, production and distribution of medical devices. ISO 13485 is a requirement for medical device distribution in Singapore, and together with Health Sciences Authority approvals, this certification made Nightingale Health ready to launch its blood testing service in Singapore.
- The opening ceremony of Nightingale Health's laboratory in Singapore was held on 2 December 2024. The opening of the Singapore laboratory marked an important milestone in Nightingale Health's global expansion. Located in the hub of Southeast Asia, the laboratory is ready to serve healthcare and medical research customers in the region. The first healthcare samples are expected to arrive to the lab in the first quarter of 2025.
- Nightingale Health and Boston Heart Diagnostics Corporation ("Boston Heart") successfully completed the pilot announced in June 2024 and signed an agreement to start providing Nightingale's blood test based Health Check to Boston Heart's customers. The parties have agreed on a commercial service model that makes Nightingale Health's blood test based Health Check widely available for Boston Heart's customers.
- Nightingale Health announced a partnership with Phenome Health, a U.S.-based non-profit research organization. The collaboration includes research studies, population health programs, and healthcare initiatives.
- Nightingale Health attained UKCA (UK Conformity Assessed) marking under the UK Medical Devices Regulations. The UKCA marking shows compliance with the UK requirements for medical devices and is an important step in bringing Nightingale Health's blood analysis technology to healthcare use in the UK.
- Nightingale Health's peer-reviewed study was published in Nature Communications, showcasing the accuracy and performance of Nightingale Health's blood biomarker-based risk prediction models. The world's largest population study in the field includes data from more than 700,000 participants in three national biobanks from Finland, Estonia and the United Kingdom. The population study findings show that Nightingale Health's risk prediction models have better risk detection capabilities for chronic diseases than polygenic risk scores and they add value beyond existing clinical risk assessment tools.
- The Board of Directors of Nightingale Health decided to investigate the possible transfer of the company's publicly traded series B shares from Nasdaq First North Growth Market Finland to the main market of Nasdaq Helsinki Ltd and the possible cross-trading of the Shares on the OTCQX market, managed by the OTC Markets Group Inc. in the United States. The aim of the possible transfer to the Main Market in Finland and entry to the OTCQX market in the United States is to improve the liquidity of the Shares and to achieve a broader international shareholder base.
Key figures (IFRS)
EUR thousand | Group 7-12/2024 | Group 7-12/2023 | Group 2023-2024 |
Revenue | 2,308 | 1,715 | 4,358 |
EBITDA | -4,723 | -5,336 | -10,434 |
Operating loss | -9,131 | -9,306 | -18,592 |
Net loss for the period | -8,202 | -8,529 | -17,463 |
Equity ratio | 94% | 94% | 92% |
Net debt to equity ratio | -77% | -76% | -76% |
Balance sheet total | 80,979 | 97,040 | 90,840 |
Number of employees on average | 92 | 85 | 84 |
Employee benefits* | -3,990 | -4,238 | -8,783 |
Net cash at the end of the period | 58,010 | 69,054 | 63,401 |
* Employee benefits include expenses in accordance with the IFRS 2 Share based payments standard, which totaled EUR 0.97 (1.49) million in the half-year period.
Share performance indicators*
Group 7-12/2024 | Group 7-12/2023 | Group 2023-2043 | |
Earnings per share (EPS), undiluted and diluted**, EUR | -0.14 | -0.14 | -0,29 |
Equity per share, EUR | 1.24 | 1.48 | 1.36 |
Market value of the shares at the end of the period, EUR | 173,808,458 | 67,340,042 | 138,783,240 |
Number of shares at the end of period | 60,928,079 | 60,918,459 | 60,918,459 |
Average number of shares | 60,919,452 | 60,918,459 | 60,918,459 |
B shares | |||
Number of shares at the end of period | 40,302,841 | 39,153,970 | 40,040,415 |
Average number of shares | 40,156,493 | 39,152,334 | 39,219,676 |
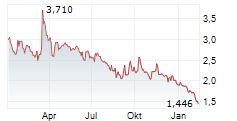
Lowest share price, HEALTH, EUR | 1.97 | 0.80 | 0.80 |
Highest share price, HEALTH, EUR | 4.40 | 1.28 | 2.30 |
Closing price at the end of period, HEALTH, EUR | 2.88 | 1.12 | 2.30 |
Average daily trading volume of the share | 93,211 | 57,903 | 59,535 |
Relative share trading volume, % | 29.37 | 18.8 | 38.0 |
A shares | |||
Number of shares at the end of period | 19,526,438 | 20,585,924 | 19,779,244 |
Average number of shares | 19,663,833 | 20,585,924 | 20,541,843 |
EMP shares | |||
Number of shares at the end of period | 1,098,800 | 1,178,565 | 1,098,800 |
Average number of shares | 1,099,127 | 1,180,201 | 1,156,940 |
* The table does not show the P/E ratio because it is negative.
** The company's potential dilutive instruments consist of stock options. As the company's business has been unprofitable, stock options would have an anti-dilutive effect and therefore they are not taken into account in calculating the dilutive loss per share. Thus, there is no difference between the undiluted and diluted earnings per share.
From the CEO
Prevention of chronic diseases is the foundation of better healthcare. While the treatment of diseases must continuously improve, chronic diseases are persistent in nature, and therefore treating those already sick does not reduce the number of people suffering from these diseases.
At the core of a preventative healthcare system is the ability to detect disease risks. When people at highest risk of disease can be identified in time, healthcare services and interventions can be targeted to those who benefit the most from them, and the onset of chronic diseases can be prevented.
Steering our society towards a healthier future means that we need to start systematically identifying disease risks. The actions and tools for reducing the risks are already well known. However, the most significant barrier to implementing preventative healthcare has been the inability to detect disease risks in a healthy population, as the risk assessment tools currently used in healthcare require too many resources to be applied on a population-wide scale.
The risk detection test developed by Nightingale Health enables, for the first time, the comprehensive and cost-effective detection of chronic disease risks at the population level. The more systematically risks are detected, the more effectively preventative measures can be targeted, thereby reducing the number of sick people in the future.
Through collaboration with Terveystalo, Nightingale Health's risk detection test is now in nationwide use in Finland, covering 30% of the Finnish workforce. By the end of 2024, over 100,000 of Terveystalo's occupational health customers had received their risk report, and more than 1,000 healthcare professionals were utilizing the risk reports in their daily patient care.
After the successful launch of the Terveystalo collaboration, we have focused on implementing the next commercial projects during the first half of the current financial year, particularly in Singapore and the United States.
In Singapore, since late 2023, we have worked together with Innoquest Diagnostics, a leading clinical diagnostics service provider, to bring Nightingale Health's blood analysis technology to Southeast Asia. During the half-year period, we obtained all the required certifications and regulatory approvals; our Singaporean subsidiary was granted ISO 13485:2016 certification and we received the necessary regulatory approvals for healthcare use from Singapore's Health Sciences Authority. These regulatory approvals cover individual blood values such as lipids, fatty acids and amino acids, which required that Nightingale Health demonstrated that the analytical performance of these blood values matches established analysis methods. I am very proud that we have once again demonstrated that we can manage different regulatory processes in different environments efficiently and with excellent results.
In parallel with the regulatory processes, we have also been setting up the laboratory and data processing readiness in Singapore, and that work was successfully completed in December 2024. The laboratory is now ready to serve healthcare and medical research customers in the Southeast Asia region. Opening the laboratory in Singapore marked an important milestone in our global expansion strategy.
In the United States, Nightingale Health and Boston Heart Diagnostics Corporation successfully completed the pilot announced in June 2024, and signed an agreement to start providing Nightingale's blood test based Health Check to Boston Heart's customers. We are very pleased with the positive reception of Nightingale's Health Check in the pilot phase. After the pilot phase, we have focused on preparing for the production phase of the service, and the work has progressed well. Nightingale's Health Check is the world-leading solution in detecting risks for chronic diseases, and we strongly believe that many customers in the United States will greatly benefit from the advantages of our technology in building more preventative, scalable, and efficient healthcare solutions.
In the second half of the financial year, we will, step by step, continue to expand the commercial use of our blood analysis technology globally. In the healthcare market, this will take time, but it will ultimately enable the creation of a global preventive healthcare system that can help in steering away from the current negative trend in healthcare. With Nightingale Health's blood analysis technology, a future with fewer sick people, more healthy life years, lower costs, and increasingly effective healthcare is achievable.
Teemu Suna
CEO and Co-founder, Nightingale Health Plc
Business targets for the financial year 2024-2025
Nightingale Health's business targets for the financial year 2024-2025 are:
- Win new large international deals
Nightingale Health aims to win new international flagship deals and convert pilots to commercial contracts to accelerate the adoption of Nightingale Health's technology in large-scale healthcare use
- KPI: Win a large-scale international healthcare project
- Increase revenue
Nightingale Health aims to continue increasing its revenue, despite the fact that new deals typically take more than 12 months to ramp-up and convert into revenue
- KPI: Increase revenue compared to previous financial year
- Improve efficiency
Nightingale Health will continue investing in growth while preserving the strong cash position and solid runway
- KPI: Improve adjusted EBITDA* level compared to previous financial year
*Adjusted EBITDA = EBITDA - share-based payments - extraordinary items - items affecting comparability
Mid-term and long-term business targets
Nightingale Health's mid-term and long-term business targets remain the same.
Mid-term business targets are:
- To conclude an agreement to analyze two million samples annually in Europe
- To conclude an agreement to analyze ten million samples annually in the United States or in Asia
- To extend laboratory capacity in respective geographical areas to meet the analysis capacity required by the aforementioned agreements
- To achieve positive EBITDA
Long-term business targets are:
- To analyze 100 million blood samples from partnerships with the healthcare sector, health initiatives, and white label partners
- To generate EUR 500 million in annual revenue from partnerships with the healthcare sector, health initiatives, and white label partners
Significant events after the end of the period
- On 23 January 2025, Nightingale Health announced the performance metrics of its latest generation disease risk assessments to be rolled out in Singapore and the United States.
- On 27 January 2025, Nightingale Health announced the collaboration with Enigma Genomics to sell Nightingale Remote Health Check in the MENA region.
- On 7 February 2025, Nightingale Health announced its updated regulatory plan in the United States.
- On 27 February 2025, Nightingale Health announced the development of innovative LLM based tool to empower anyone to take informed decisions to lower disease risks.
- In addition, Nightingale Health has, as part of its effort to establish a laboratory in the Unites States, committed to a lease of a laboratory and office space in the Unites States.
Live webcast for investors and media
Nightingale Health will arrange a live webcast for investors and media in English on 6 March 2025 at 2 p.m. EET. The webcast can be followed online at:
https://nightingalehealth.events.inderes.com/2024-2025-h1-results
Presentation will be held by CEO Teemu Suna and CFO Tuukka Paavola. A recording of the event will be available later the same day at www.nightingalehealth.com/investors.
Helsinki, 5 March 2025
Nightingale Health Plc
Board of Directors
For further information, please contact:
Teemu Suna, CEO
ir@nightingalehealth.com
Certified Adviser:
Oaklins Finland Ltd, tel. +358 9 6129 670
About Nightingale Health
Nightingale Health's mission is to build sustainable healthcare and reduce health inequalities. Nightingale Health has developed the world's most advanced health check that provides risk detection for multiple chronic diseases from a single blood sample. Nightingale's Health Check can be scaled to entire populations at a low cost, and it can replace many of the current clinical risk assessments. Detecting disease risks on a population level allows for the effective targeting and tracking of health interventions, and better prevention of the onset of chronic diseases. With every sample we help to create a healthier world.
Nightingale Health operates globally with a parent company in Finland and eight subsidiaries in countries such as Japan, the United States, Singapore, and the United Kingdom. Nightingale Health has customers in more than 34 countries in the healthcare and medical research sectors. The company's technology is being used in many of the world's leading health initiatives, such as the UK Biobank, and over 600 peer-reviewed publications validate the technology. The company's Series B shares are listed on the First North Growth Market Finland marketplace. Read more: https://nightingalehealth.com/