WASHINGTON (dpa-AFX) - Zimmer Biomet Holdings, Inc. (ZBH), Friday announced that the company has received the U.S. Food and Drug Administration 510(k) clearance of Persona Revision SoluTion Femur, a revision knee implant component offering an alternative for patients with sensitivities to certain metals.
The company explained that the Persona Revision SoluTion Femur is made solely of a proprietary Tivanium alloy, which is treated with the Ti-Nidium Surface Hardening Process.
Persona Revision SoluTion Femur will be commercially available in the country in the third quarter of 2025.
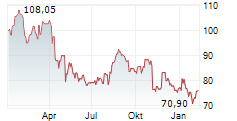
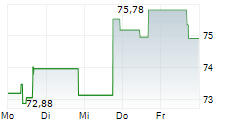
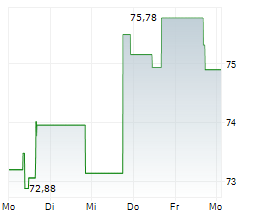
In the pre-market hours, Zimmer's stock is trading at $105.05, down 0.26 percent on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News