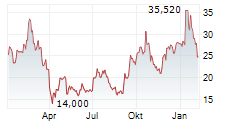
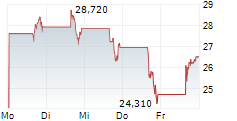
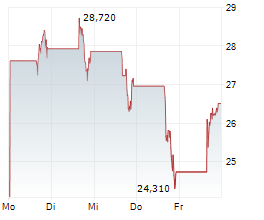
WASHINGTON (dpa-AFX) - Beam Therapeutics Inc. (BEAM) Monday reported positive initial data from its Phase 1/2 study of BEAM-302 for the treatment of alpha-1 antitrypsin deficiency (AATD).
AATD is an inherited genetic disorder that affects the lungs and/or liver, leading to early onset emphysema and liver disease
BEAM-302 is being evaluated in a Phase 1/2 study to evaluate its safety, tolerability, pharmacodynamics, pharmacokinetics and efficacy. Part A of the trial is designed to evaluate AATD patients with lung disease, and Part B will evaluate AATD patients with mild to moderate liver disease with or without lung disease.
Preliminary results from the first three single-ascending dose cohorts showed that BEAM-302 was well tolerated, with single doses of BEAM-302 leading to durable dose-dependent correction of the disease-causing mutation. Further, no serious adverse events were reported.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News