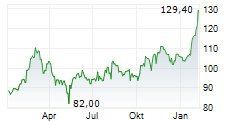
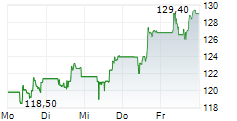
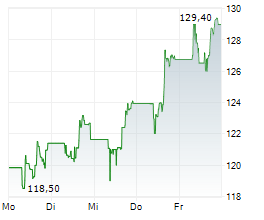
FOSTER CITY (dpa-AFX) - Gilead Sciences, Inc. (GILD) Wednesday said it presented encouraging long-term data from ALLIANCE study.
ALLIANCE is an ongoing Phase 3 study to evaluate Biktarvy versus dolutegravir plus emtricitabine/tenofovir disoproxil fumarate F/TDF, DTG+F/TDF, in adults with HIV-1/HBV co-infection initiating treatment.
New data from the study showed that Biktarvy maintained 95.4% of HIV-1 and 86.6% of HBV virologic suppression in participants following a switch to Biktarvy after 96 weeks of treatment with DTG+ F/TDF.
These data were presented at the Conference on Retroviruses and Opportunistic Infections (CROI 2025).
Additionally, the company said its Phase 2 study of lenacapavir with teropavimab and zinlirvimab (LTZ) met its primary goal of proportion of participants with HIV-1 RNA - 50 copies/mL at Week 26.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News